INTRODUCTION
Randomized Controlled Trials1,2 can comprise several different designs. As is usual, the subjects in this study were randomly assigned to treatment or placebo groups, the subjects were blinded to whether they received a treatment or a placebo and the providers of the treatment or placebo did not know the patient’s condition. However, the providers could not be blinded to whether they were adjusting the occlusion or implementing a placebo, making this a single blind study. The assessors of the success or lack of success of the treatment or placebo, did not know which patients received the treatment or the placebo.
TMD can be characterized by frequent temporal headaches, neck pain, ear pain, chewing fatigue, clenching and grinding habits (bruxism), morning muscle stiffness, limited mouth opening, with or without clicking or popping sounds within the Temporomandibular Joints.3–6
The role of occlusal adjustment in the reduction of chronic muscular pain symptoms has not been defined to the satisfaction of all.7 In a few controlled clinical occlusal adjustment studies the treated subjects showed dramatic improvements in symptoms,8–10 while other studies using different occlusal adjustment techniques could not find a correlation between their occlusal corrections and muscular pain symptoms.11–13 Occlusal adjustment studies also differ from studies that document occlusal anatomic factors (overbite, underbite, crossbite, Class I, II & III, etc.) that may or may not be correlated with TMD symptoms. No strong correlations have been found between visible anatomic occlusal components like overbite and underbite and TMD symptoms.14 These observations have led to opinions that mal-occlusion does not contribute to TMD symptoms, advocating instead that TMD results from emotional problems, not occlusal factors.15
Contrary to those opinions, a recent 2018 publication utilizing the Beck Depression Inventory-II (BDI-II)16 and eighty-three chronic myogenous TMD patients who underwent computer-guided occlusal adjustments that resolved their physical symptoms, also reported that the subjects’ emotional depression status dramatically resolved within 3 months post treatment.17 That study disproved the opinion that the chronic pain and dysfunction of those TMD patients were caused by emotional depression.
Observational and visually determined occlusal classification studies cannot measure the inter-arch occlusal force distribution nor the timing durations that the occlusal contacts remained frictionally engaged during excursions. However, both of these occlusal parameters are measured by the T-Scan system and each one has repeatedly been correlated to myogenous TMD symptom appearances.10,17–21
In prior unsuccessful attempts at occlusal adjustment, the authors employed conventional articulating paper-only occlusal adjustments to resolve TMD symptoms. Recent studies have shown that articulating paper marks cannot delineate which contacts bear heaviest forces, nor can they reveal occlusal contact timing order in any way.22–26 Previous corrections applied to the subject occlusions in the ineffective treatment studies have been CR to CO discrepancy removal, and balancing incline adjustments.11–13
Many authors have employed computerized occlusal analysis when treating muscular pain.5,6,10,17,18,27–31 The T-Scan 10 computerized occlusal analysis system (Tekscan, Inc. S. Boston, MA, USA) digitally records occlusal parameters quantitatively, reporting real-time occlusal contact sequencing measurements that play as a video in dynamic force movies. Studies involving T-Scan-guided occlusal adjusting have reported that muscular pain symptoms and the emotional depression that accompanies living in chronic pain, were readily resolved after ICAGD occlusal adjustments were rendered.17,32
Specifically, Disclusion Time Reduction (DTR) of all molars and premolars to < 0.5 seconds per lateral excursion has been shown to reduce muscle hyperactivity levels and their related myogenous symptoms pre to post treatment. Different authors using this technique have reported statistically significant differences in Disclusion Time durations, muscle contraction levels, Time-to-Muscle Shutdown durations, and rapid muscular TMD symptom resolutions.5,6,17–19,26–32 The Disclusion Time is reduced using a T-Scan guided and measured occlusal adjustment procedure known as Immediate Complete Anterior Guidance Development (ICAGD), wherein posterior excursive occlusal interferences are removed selectively from all working and balancing molar and premolar contacts, until the Disclusion Time value becomes < 0.5 seconds per excursion.27Although only one ICAGD controlled occlusal adjustment study has been performed to date,10 there has been only one other Randomized Controlled Trial occlusal adjustment study33 in the past 50 years and one “mock” equillibration.34
OBJECTIVES
Previous TMD, non-occlusal adjustment, supportive therapy treatment randomized clinical trials have reported statistically significant placebo effects, despite the use of a sham therapy.35–37 The Objectives of this study were; 1) to perform a Disclusion Time Reduction ICAGD study with a single-blind, placebo-controlled RCT design, 2) to either corroborate or contradict the observed symptom reductions of the prior, non-RCT Disclusion Time Reduction studies and 3) to measure any placebo effect.
METHODS & MATERIALS
From Rajiv Gandhi University of Health Sciences Dental College, 236 1st year dental students filled out a questionnaire to qualify them for possible study participation. Only 1st year students were sought out, as they were not known by the research team in any student–doctor relationship, and as 1st year students, they were naive to clinical procedures like occlusal adjustment.
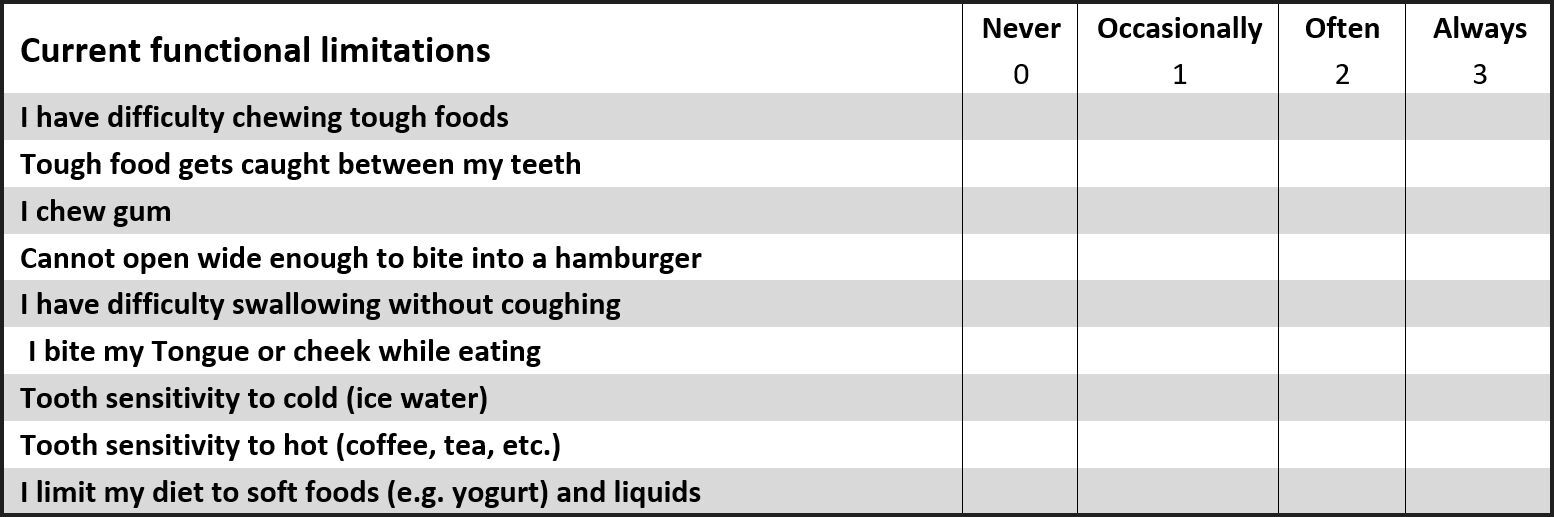
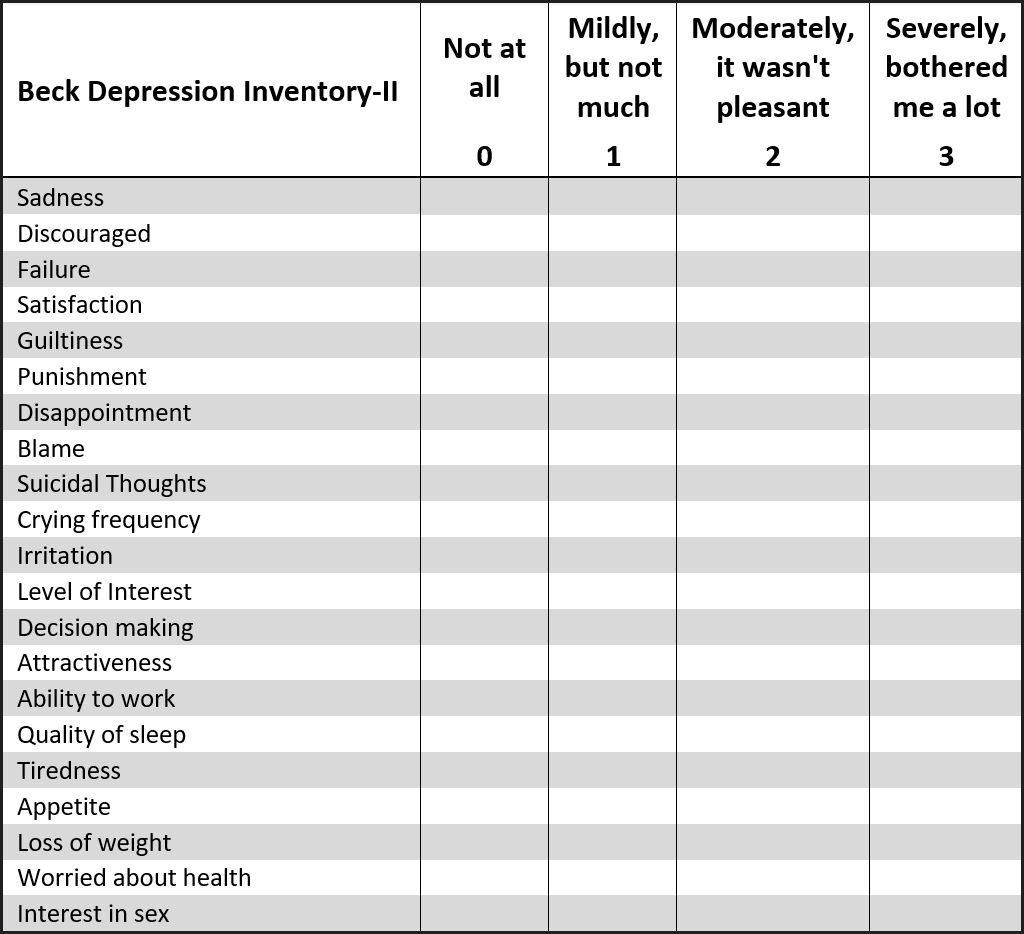
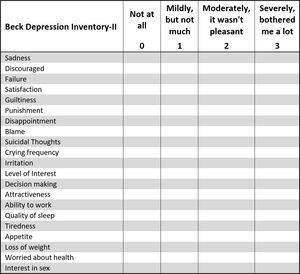
The questionnaire asked about the student’s history of TMD symptoms, any treatments for TMD they may have received in the past, and the frequency and intensity of their common TMD symptoms that they currently experienced. Each potential subject also had completed a Beck Depression Inventory (BDI–II) sheet,33,38 which classified their levels of emotional depression related to living with TMD symptoms.
The Inclusion criteria were:
-
A history of reported muscular myofascial pain dysfunction symptoms.
-
A fully dentulous state of at least 28 teeth
-
Near normal occlusal relations with molars and premolars in contact excursively in right and left excursions.
-
Angles Class I and Class III subjects with guiding teeth that were either in contact, or near to contact.
-
Patients younger than 26 years to create homogeneity within the subjects
The Exclusion criteria were:
-
Severe Class II and Class III mal-occlusions and large anterior open occlusions, where anterior guidance contacts could not exist
-
A previous history of trauma to the TMJ region or unstable internal derangements.
-
Patients younger than 19 years
-
Patients who had undergone any prior treatment for Temporomandibular Disorders including any prior occlusal adjustment treatments
Ultimately, all of the selected 1st year dental students demonstrated medium to high Beck Depression Inventory-II scores, presented with an Angle’s Class 1 skeletal relationship and were experiencing muscular TMD symptoms. These subjects’ names were placed in a hat and then a non-treating researcher (SP) randomly allotted them into a treatment group or a control group, non-alphabetically, by pulling their names out of the hat. The order their names were chosen determined their group placement. The odd numbers (1st, 3rd, etc. names) were given the ICAGD treatment, the even numbers (2nd, 4th, etc. names) received the placebo treatment.
All included subjects filled out an informed consent that stated the participant subjects would not be told if they were to receive the treatment or the placebo, but that both groups would have their enamel adjusted and polished. The subjects were never advised of the difference between a non-treatment with a polishing cup and the real ICAGD. Therefore, all participating subjects in both the control and treatment groups expected to undergo a real occlusal adjustment procedure.
Treatment Procedure Vs Placebo Procedure
To further blind all subjects from which treatment they would receive, the dental handpiece was covered by a cloth that hid the bur or polisher to be used from the subjects’ view. The protocol was reviewed and approved by the IRB of the Health University RGUHS (Rajiv University of Health Sciences).
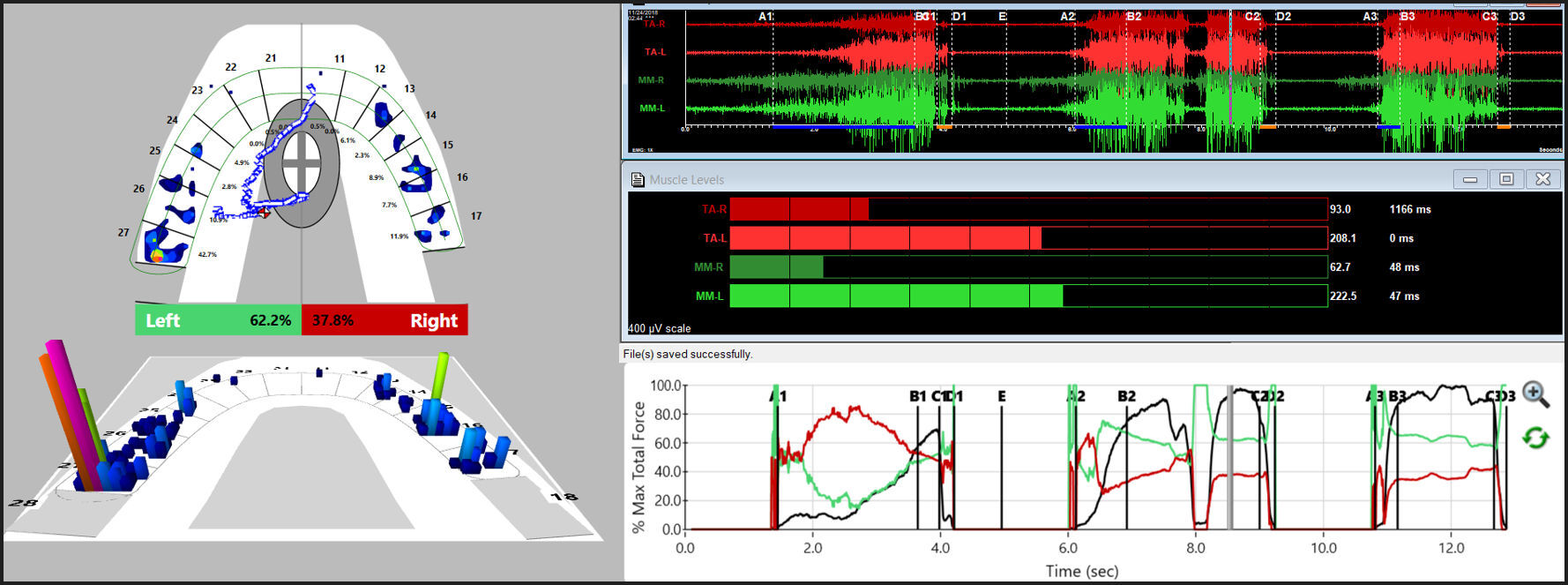
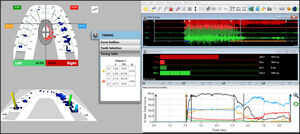
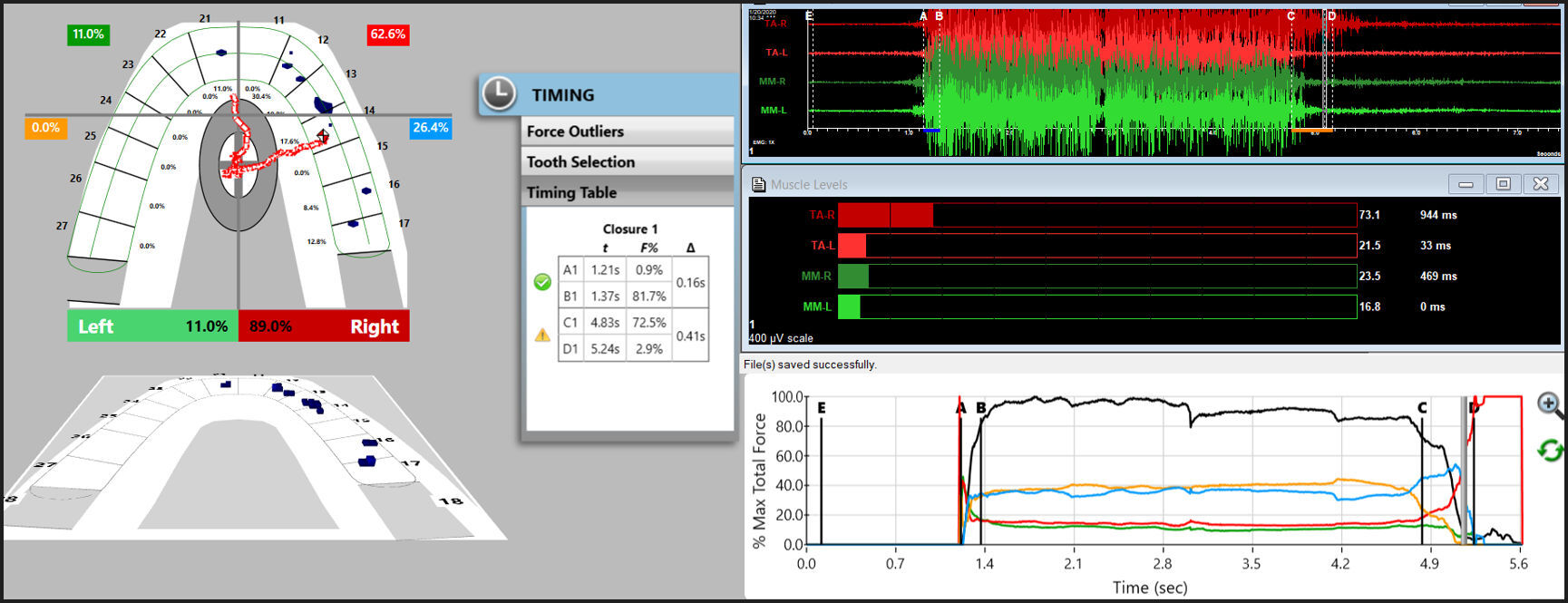
The synchronized T-Scan/EMG technologies (Figure 1) were used to record the right and left excursions of all subjects closing into their Maximum Intercuspal Position (MIP) firmly, holding their teeth together for 1-3 seconds, and commencing a right or left excursion until only anterior teeth were in contact. This specific recording method insured high quality Disclusion Time and EMG data was obtained from the subjects.32 (Figures 1, 2a and 2b).
All subject’s pre-treatment Disclusion Time values and excursive electromyography levels were recorded for comparison to the post ICAGD coronoplasty, and the post placebo polishing Disclusion Time values and EMG levels in the Treated and Control subjects, with the below schedule:
-
Treated group - Pre-treatment = Day 1, then at 1 week and 1month, post-treatment recordings were obtained as subjects underwent the ICAGD procedure. At the 3 months and 6 months recall visits, only a single set of measurements were made, and no further ICAGD treatment was performed.
-
Control group – Pre-treatment (placebo) = Day 1, then at 1 week and 1 month, pre and post-placebo recordings were obtained as subjects underwent the simulated ICAGD procedure. At the 3 months and 6-month recall visits, only a single set of recordings were made, and no placebo polishing was performed.
Occlusal Adjustment Procedures that were Performed on the Treated Group
The ICAGD definitive occlusal adjustments were accomplished in 2 phases:
- Phase I - Excursive Interference Adjustments
The subjects’ teeth were air dried. The patients were then asked to close into their Maximum Intercuspal Position (MIP) with articulating paper (Bausch, Arti-Fol® Red, 8μ, Germany) interposed between their teeth, and then to commence a right mandibular excursion out to edges of their canine teeth, then slide back into MIP, and then make a left mandibular excursion out to the edges of the left side canine teeth, and back into MIP (Figures 3A - 3C).
The pre-treatment T-Scan/BioEMG recordings (Figures 2A and 2B) guided the clinician to the areas to adjust, where prolonged excursive frictional contacts were located on inclined planes and on outer aspects of the buccal cusps (Figures 3D and 4A). These excursive contacts were then eliminated on all involved surfaces using pear-shaped finishing burs (Mani Dia-Burs, Japan ISO no-237/021) bilaterally, leaving the central fossa, cusp tip, and the marginal ridge contact points intact (Figure 4B).
ICAGD was considered completed when:
-
All Class I, II, and III lateral posterior excursive interferences had been visually removed
-
Disclusion of all posterior teeth in the right and left excursions was visible, with the patient experiencing easier lateral movements than pre-ICAGD
-
All lace-like linear contacts had been removed
-
The remaining pattern of habitual closure contacts were located solely on cusp tips, fossae, and marginal ridges (Figure 4B).
Then new excursive recordings were obtained to verify the Disclusion Times had been reduced to < 0.5 seconds in each excursion. Any remaining prolonged excursive contacts were adjusted until each excursion’s Disclusion Time was less than 0.5 seconds (See Figures 5A and 5B).
- Phase II - Habitual Closure Adjustments to the new MIP. Once all the four posterior quadrants had been properly treated with ICAGD, subjects were then asked to make unguided mandibular closures into their Maximum Intercuspal Position (MIP). Any rapidly rising forces detected by the T-Scan and any high points perceived by the patient were refined, to achieve measurable bilateral occlusal force balance and patient comfort.
When only solid areas of contact in MIP remained and the T-Scan’s Center of Force trajectory rested near the T-Scan arch-half midline (indicating near equal right side-left side occlusal force arch half percentages), the habitual closure adjustments were completed (Figure 6).
2nd and 3rd Treatment Appointments (Day 7 and Day 14)
At days 7 and 14, each treated subject filled out five new (post ICAGD) questionnaires; for BDI-II, Pain levels, Frequency of Symptoms, Functional Restrictions and Frequency of Pain. Then, new habitual closure into MIP T-Scan/BioEMG recordings were made, which guided further MIP closure adjustments. These adjustments were followed by a second set of T-Scan/BioEMG recordings that measured the final occlusal force percentage balance (or imbalance), the Disclusion Times, and the EMG levels present in each excursion. This completed the treatment phase.
Procedures Performed for The Placebo Group - Mock ICAGD
Pre-polishing and post-polishing ICAGD, the Disclusion Time T-Scan/BioEMG recordings were made in the same fashion as was done with the treated group. The patients also performed excursions and unguided closures through the articulating paper in the same fashion as did the treated group. The resultant excursive contact patterns were polished with a rubber cup and polishing paste 3-5 times per arch, so as to mimic the true adjustments performed on the treated subjects.
Habitual closure placebo adjustment phase
The habitual closure contact patterns were also polished 3-5 times per arch, until the patients reported that they “felt” their polished contacts were balanced and comfortable. There is no way to know for certain, but some subjects may have reported improvement just to get the process over with or they may have been fooled into believing an improvement in their occlusion had been accomplished.
The placebo ICAGD group had their Disclusion Time T-Scan/BioEMG III data recorded once per day on days 7 and 14, and at the 3 and 6-month recall intervals. At each date the placebo adjustment subjects reassessed their symptoms by filling out five new questionnaires; BDI-II, Pain levels, Frequency of Symptoms, Functional Restrictions and Frequency of Pain. Figures 7A, 7B, 8A and 8B show that the control polishing did not change the occlusal balance, excursive function, or the muscle physiology from one measurement visit to the next.
All treated and control subject self-assessment data by appointment (the repeated BDI-II, Pain Scale, Symptom Frequency, Functional Restrictions and Frequency of Pain questionnaires), were subjected to the non-parametric Mann-Whitney U Test. The Disclusion Time values pre and post ICAGD or mock ICAGD, and the excursive EMG levels pre and post ICAGD or mock ICAGD, were subjected to the Students paired t-test (Alpha < 0.05).
RESULTS
The Control Group
Although the control and treatment groups were selected by a random process, they were not found to be completely equal. The mean age of the control group (18.6 yrs. +/- 1.12), compared to the mean age of the treatment group (21.1 yrs. +/- 1.83), was significantly younger (p < 0.05). Random selection does not guarantee equality in all aspects. However, with the very limited age range of these subjects, it is unlikely that this age difference could have confounded the results.
The progression of mean self-assessment scores obtained from the control group did not reveal the classic pattern associated with a placebo effect, but instead were in part the reverse of an expected placebo response (p < 0.01). See Table 1.
-
The mean Beck Depression Inventory-II scores increased significantly from pre-treatment to week one post placebo treatment (p = 0.0099), but then stabilized throughout the six-months study duration.
-
The mean Functional Restriction scores initially increased significantly (p = 0.00001), and then remained constant throughout the six-months study duration.
-
The mean Pain Scale scores, Symptom Frequency scores and Frequency of Pain scores did increase slightly compared to pre-placebo, but did not change significantly from pre-placebo throughout the six-months study duration (p > 0.05).
The Treatment Group
The Treatment Group, being significantly older, reported significantly higher pre-treatment self-assessment means for their Pain Scale, Symptom Frequency, Frequency of Pain Symptoms (p < 0.01). See Table 2. However, their Beck Depression Inventory-II scores and their Functional Restriction scores were not significantly different from the control group prior to receiving treatment (p > 0.05).
The treated subjects demonstrated:
-
A dramatic reduction in all five self-assessment scale means at one week after ICAGD (p < 0.00001).
-
A further significant decrease in the self-assessment means of all five scale means at one-month (p < 0.00001).
-
A final significant decrease in the self-assessment means of four of the scale means at three-months (p < 0.0130).
-
No further significant changes in the five self-assessment scale means were reported at six-months (p > 0.05).
DISCUSSION
The Immediate Complete Anterior Guidance Development (ICAGD) is a process that reduces the frictional contacts of the posterior teeth occurring during lateral excursive movements. The purpose of this reduction is to measure immediate disclusion of the molars and premolars with cuspid guidance. The metric that is used to determine how well this has been accomplished is referred to as Disclusion Time, which is simply the amount of time (in seconds) between the start of the lateral excursion and reaching full disclusion. Table 3 reports the discussion times for both groups pre and post treatment or placebo.
Analysis of two BDI-II subscales
The Beck Depression Inventory-II can be divided into 2 subscales, to separate cognitive depressive symptoms from somatic-affective depressive symptoms.39–42
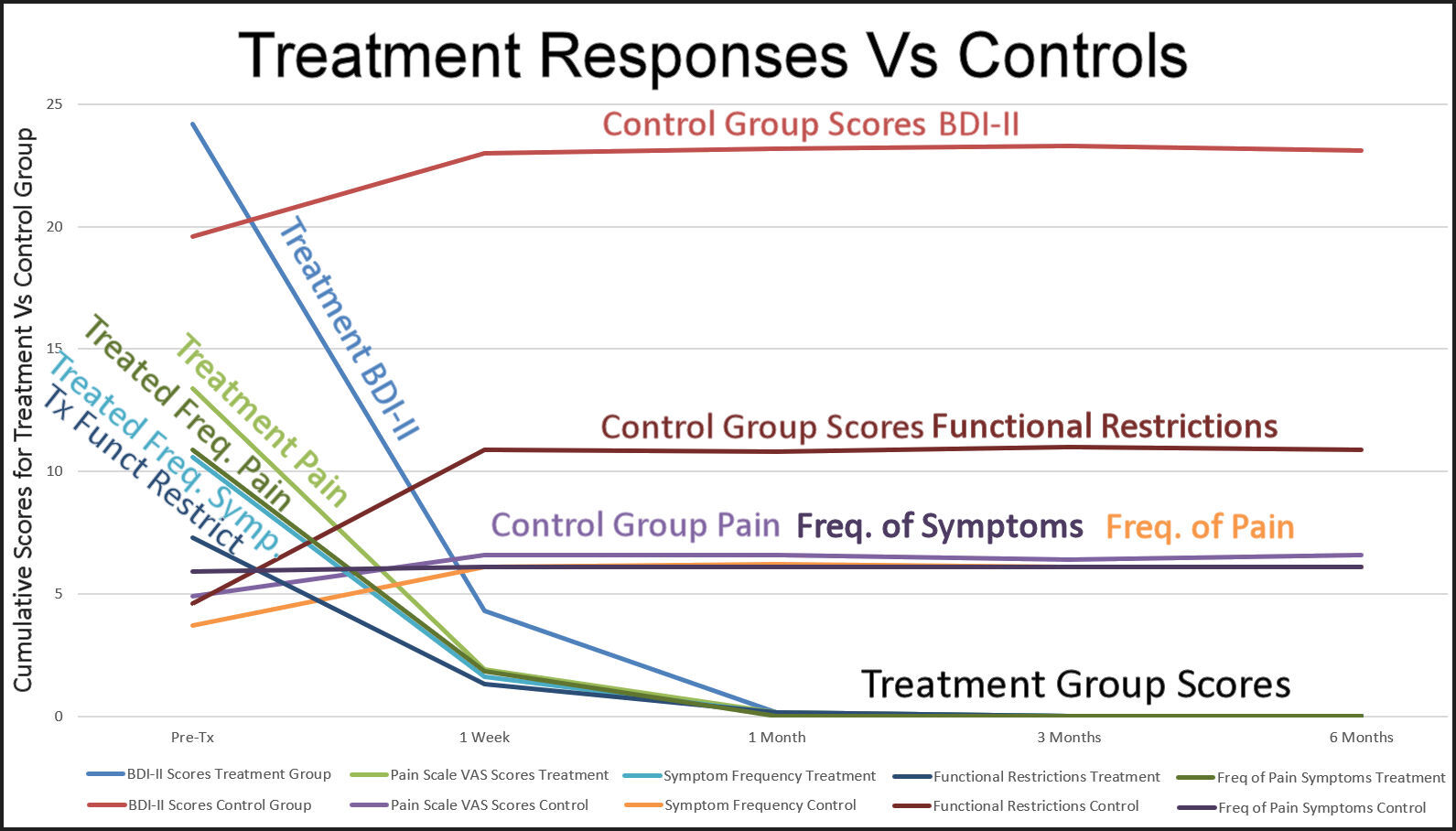
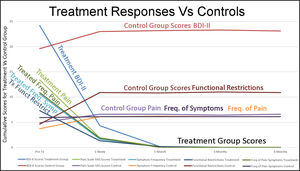
Figure 9 graphically demonstrates that after 1 week the control group self-assessment scores increased slightly, then maintained similar mean BDI-II depression scores, mean pain level scores, mean frequency of symptoms scores, mean functional restriction scores and mean frequency of pain scores to the Treatment Group before they underwent ICAGD. Starting that first week and continuing for the remainder of the study, the treatment group’s levels of depression, of pain severity, of the symptom frequency, of functional restrictions and of pain frequency declined.
Within the BDI–II, 11 of the 21 questions tend to reveal cognitive-depressive symptoms related to physical pain conditions, while 8 of the questions tend to reveal somatic-affective factors related to psychiatric conditions. The remaining 2 questions are considered neutral and are not included in a BDI-II subscale analysis.43,44
Cognitive dysfunction refers to deficits in attention, verbal and nonverbal learning, short-term and working memory, visual and auditory processing, problem solving, processing speed, and motor functioning. Further, cognitive depressive symptoms are commonly associated with a medical condition, (often, but not always), resulting from chronic pain or some physical discomfort. The somatic-affective depressive symptoms are commonly associated with a psychiatric diagnosis of a Somatic Symptom Disorder (SSD). SSD is characterized by an extreme focus on physical symptoms like pain or fatigue, that cause major emotional distress and problems functioning, where the patient’s symptoms constitute an over-reaction.
For both the treated and control subjects, the 11 cognitive-depressive symptom scores were totaled, as were the 8 somatic-affective depressive symptom scores. Because the cognitive-depressive symptoms include 11 of the 21 questions, and the somatic-affective symptoms include only 8 of the 21 questions, the cognitive total was multiplied by 8/11 (0.7272) to adjust it fairly to the somatic-affective total. The adjusted cognitive scores were then compared to the somatic-affective scores and found to be significantly higher (p < 0.05) (See Table 4). Although there is overlap between the subscales and no absolute definitive differences can be asserted,43,44 because most of the subjects scored in both categories, the higher mean cognitive scores suggest that the total subject pool suffered from medically painful conditions, that triggered various levels of mild to severe depression. The fact that the treated subjects responded very positively to their physical treatment reinforces that likelihood.
The Results of this ICAGD RCT study do corroborate the findings of previous non-RCT ICAGD studies, where marked and rapid symptom improvements were reported following ICAGD.5,6,10,17,18,27–31 This study tracked their symptom intensities and frequencies, and their emotional impact on subjects. Many painful symptoms improved within one week after ICAGD was rendered, which led to significant improvements in the subjects’ levels of depression that continued to improve over the first 3-months of observation. The results also strongly indicate that the placebo treatment was not convincing enough to the control subjects, to initiate a conventional or expected placebo effect. With the lack of change to the control group’s Disclusion Times by not undergoing real ICAGD, the placebo ICAGD treatment did not induce any patient perception that they had received “a potentially corrective treatment” for their TMD symptoms.
The placebo effect is considered real, in that it has been observed in some medical studies35–37 and in a limited number of occlusal adjustment studies.33,34 However, it apparently does not always occur, as in this ICAGD study, where initially, the reverse effect was observed. The control group as a whole became more symptomatic, and remained more symptomatic for 6 months after the placebo treatment was rendered.
The lack of any positive placebo effect from the control group may be reflective of the difficulty of applying an effective faux occlusal adjustment treatment. As often happens, it was also not possible to blind the operator, resulting in a single blind design. It is possible that some of the subjects in the control group may have realized that the faux occlusal treatment was just that, or the faux occlusal treatment may have actually irritated some of the subjects in the control group resulting in a reverse placebo effect (negative instead of positive). The significant increases in the control subjects’ initial Beck Depression Inventory-II mean scores and the functional restriction mean scores may have been due to the worsening of their physical conditions over time, as the placebo treatment did not in any way improve their occlusal contacts, nor improve their Disclusion Times. Disappointment with respect to the faux occlusal adjustments not helping the control subjects physically, was reflected in an increase of the control group’s mean Beck Depression score.
Alternatively, the treated subject’s immediate, significant and dramatic reductions in all five Pain, Functional Restriction and BDI-II scores, corroborated the previous studies’ conclusions of effectiveness, especially when the ICAGD Coronoplasty is performed by highly trained practitioners. Although at one-week post-ICAGD, the symptoms were not completely resolved, further significant reductions were reported by the treated subjects at one month and three months, without any further ICAGD treatment being rendered after the 14th day. At the 6-month re-test no further significant reductions were found, indicating that ICAGD rapidly impacts the patient’s physiology once the Disclusion Times have been properly shortened. This study’s results confirm those of another published study that found Temporomandibular Disorders symptoms can continue to abate for up to 3 months’ post ICAGD.15 This study’s treatment group mean self-assessment scores continued to significantly decrease until the three-month re-test, but did not further improve at 6 months.
SUBSCALES of BDI-II
Table 4 defined the Subscales from the Beck Depression Inventory-II self-assessment, where this entire TMD subject group exhibited greater adjusted cognitive depressive mean symptom scores than somatic-affective symptom scores. It is important to point out that Chronic Fatigue Syndrome (CFS), which is included within the scope of TMD, has been shown to precipitate many of the same responses to the BDI-II that somatic-affective conditions produce.43 It is likely that other TMD conditions may create similar equivocal or ambiguous responses to the BDI-II, with both cognitive and somatic affective responses due to the substantial interaction between them.44 Therefore, when diagnosing TMD patients, it is very important that the physical examination accurately reveals all existing contributory chronic pain conditions, or rules out all physical chronic pain conditions, before labeling a TMD patient psychiatrically with Somatic Symptom Disorder.45
LIMITATIONS
The lesser age of the control group compared to the treatment group may have affected the significant differences in two of the five parameters’ initial symptom levels, and possibly the observed reverse placebo effect. However, this group age limitation seemed to be inconsequential as the control group means of the four self-assessment parameters did closely approach the initial mean self-assessment scores of the treatment group after week one.
The impossibility of applying a double-blind faux occlusal treatment to the control group is always an unavoidable limitation when human treatment studies are attempted. Although the control subjects may have been unaware that they weren’t receiving definitive ICAGD treatment, the control group clinician was aware they were rendering a placebo treatment. This can theoretically result in risk of bias, where the operator knows he/she is performing the placebo treatment, and may reveal it in some way to the patient. Or the clinician may haphazardly perform the placebo.
Unlike a placebo used in drug trials, where the placebo pill looks exactly like the real medication pill, in human occlusal adjustment randomized trials it is not possible to blind the operator from which treatment they are rendering. To counter this limitation, the clinician performing the faux treatment made every effort to thoroughly blind the control subjects from seeing that the dental handpiece was loaded with a rubber cup, instead of an enameloplasty bur. The authors felt it was important to perform a faux treatment on the control group, rather than perform no treatment, or have no control group.
CONCLUSIONS
Chronic painful muscular TMD symptoms, functional restrictions, and the resultant levels of emotional depression from living with chronic painful symptoms, were all dramatically improved within the treatment group within weeks after they underwent ICAGD. Symptom improvements were maintained over the 6-month period of observation. However, the placebo polishing was unable to initiate a placebo effect within the control subjects. Because polishing made no physical changes to the control group’s Disclusion Times, the prior-to-polishing excursive contact friction, and the resultant symptom-causing excursive muscle hyperactivity also remained unchanged, perpetuating and maintaining the control groups’ TMD symptoms.
CLINICAL SIGNIFICANCE
This study confirms that computer-guided Disclusion Time Reduction Therapy (DTR) is also a rapid and effective neurologic treatment approach for a wide range of painful, chronic, physical and emotional TMD symptoms, who’s therapeutic improvements are real, and not brought on by an emotional “hope” that it will “work”. Clinically, DTR effects changes within the CNS because of specific, measured, computer-guided changes that the teeth undergo. DTR simplifies treatment for appropriate TMD patients because it is performed in Maximum Intercuspation (MIP), requiring no appliance, deprogramming, or mandibular repositioning (out of MIP) to effect positive patient outcomes. This makes DTR self-limiting, as there is no need to re-occlude the teeth orthodontically or prosthodontically after an orthosis has fully altered the patient’s vertical dimension.
Potential conflict of interest statement
The corresponding author is a consultant to Tekscan, Inc., but receives no monetary or other gain from the sale of product. JR is Chairman of the board of Directors of BioResearch Associates, Inc.
Funding statement
Funding for this RCT study was provided by RGUHS- Rajiv Gandhi University of Health Science, 32nd E Cross Rd, 4th T Block East, Pattabhirama Nagar, Jayanagar, Bengaluru, Karnataka 560041, India.

_in_maximum_i.jpg)






_trajectory_turn.jpg)











_in_maximum_i.jpg)





_trajectory_turn.jpg)