INTRODUCTION
The inclusion criteria for this article were reliant upon previously published scientific studies. Excluded were editorials as well as conversations or publicly known emails between dental professionals. Human subjects in vivo were chosen for their reports and findings within this article.
The presence of Cervical Dentin Hypersensitivity (CDH) associated with occlusal overloading was reported in the literature by Kornfeld in 1932.1 It was termed at that time as “cervical erosion” in contradistinction to decay. Kornfeld advanced a concept that decay progresses in a conical fashion from outer enamel or dentin regions with its base toward pulp whereas the erosive lesions have placement of the base toward the oral environment. He stated that “sensitiveness” (sic) which existed during erosive progression resolved within a week to 10 days following minimal bite/occlusal adjustment (coronoplasty). He did not report objective means for detecting this clinical sensitivity finding nor the “minimal amount” of “bite adjustment” treatment.
Literature over subsequent decades finds the term of “dentin hypersensitivity” (DH) used to describe this “sensitiveness”, but it was not until the 1990’s when the more definitive term cervical dentin hypersensitivity appeared.2,3 Clinical distinction finds that dentin sensitivity is reported as a distinctly different pain than CDH. Post- operative dentin sensitivity following restorative placement is characterized as a dull and more slowly reactive nociception (pain) induced from stimuli similar to those producing CDH, even though dentin tubules are occluded by these materials.4 Of clinical interest is that an explorer, periodontal probe, or air do not produce a rapid nociception whereas CDH does/can. A threshold of open dentin tubules due to cementum, smear, and/or pellicle layer loss from cervical root surface regions has been implicated during the development of CDH.5–8 Pashley stated in 1990 that a diagnostic means needed to be developed with a rapid short- duration of air exposure to cervical regions for minimizing evaporative effects of tubular dentin liquids.5
The primary difference between smear and pellicle layer is that a smear plug occludes dentin following “cutting” procedures, whereas pellicle is glycoprotein and other salivary deposits upon open tubules.9
Shore indicated in his 1976 text, the complex nature of inter- relationships between pulp and periodontal neurosensory information, muscles of mastication, as well as Temporomandibular (TM) joint responses to occlusal force. He did not differentiate DH from CDH, but reported cold sensitivity reduction, a pulp pain signal to teeth following occlusal therapy.10 His histological description of cementum and bone degradation as well as secondary dentin formation over time is an effect of loading forces from compressive and tensile stress in the cervical region.
Emling’s publication in 1982 cites extensive archeological history of CDH dates from the 1757 reporting by Shaeffer.11 Due to dental terminology existing in earlier times, as well as their definitions, it is not certain whether Shaeffer reported on CDH since this term was of more recent use.12 Emling did not introduce terminology trends or definitions in the 1980s, but rather discussed historical concepts for “sensitive teeth”. His literature review reported primarily historical methods and materials to obtund this chronic pain signal from pulp. Interestingly, the henbane plant had been reported from as early as 1200 A.D. to reduce nociception sensitivity with burned seed application. Pain relief from powder of newts, lizards, and beetles, applied with a right hand only, were deemed effective for this dental pain relief. Emling further stated that these treatments were of “true empirical science” since no rationale for cause and effect could be established by investigative instruments available at respective periods of time. Of note, is that we can only assume that this later powder mixture was advanced to relieve CDH symptoms due to the lack of a term and definitions for lesions in the cervical region. We rely upon currently used terms and definitions to interpret “meaning” for scientific reports or investigations.
Dentin hypersensitivity (DH) appears during vascular insult of transient increased pressure (pulpitis). A primary difference is in location and speed of pain reaction from a stimulus. Investigations are indicated to determine if this nociceptive difference between DH and CDH are a result of neurogenic mediator release (neurogenic inflammation), variant receptor innervation, or distance from pulp tissues. Cervical Dentin Hypersensitivity has been reported by modern literature to arise as a rapidly induced pain response to a stimulus from air, cold, tactile, electrical, acid exposure, or combinations of these stimuli to dentin in the cervical region.5–8,13–30 Terms of DH and CDH must be used to distinguish these terms in the literature when reporting their respective neural events of nociception.
The clinical detection by dental health professionals or from patient complaint of CDH has yielded many modalities to obtund this pain.18,22,26,31–53 Various treatments have been used in modern times including: alteration of oral hygiene methods/toothpastes, reduced acidic diet, over- the- counter (OTC) desensitizers, iontophoresis, laser therapy, or restorative treatment, to name a few.8,49,54–57 It is not clear whether open dentin tubules are the result of cementum or stress- induced smear/pellicle layer loss; friction from improper hygiene methods, biocorrosion, stress, or combinations of these mechanisms produce CDH. Figure 1 shows the Schema of Pathodynamic Mechanisms developed by Grippo and Oh in 2017 to identify all mechanisms contributing to CDH and Noncarious Cervical Lesions (NCCLs) with CDH appearing first. However, the use of OTC desensitizers or methods to occlude tubules has been found to reduce the presence of CDH. Occlusal equilibration or additive restorative treatment has been advanced to support concepts introduced by Kornfeld decades ago.1 Etiologic theories exist to explain CDH occurrence primarily from stress and/or biocorrosion. However, further investigations are indicated to verify their contribution for this common chronic pain.
Mechanisms for DH/CDH
Gysi’s postulates from 1900 proposed dentin fluid flow stimulation of neural components at the pulp-dentin interface.13 His concepts led to investigations by Brännström with the Scanning Electron Microscope (SEM) for dentin tubule fluids. The Hydrodynamic Theory advanced by Brännström in 1963 was based upon earlier publication in 1960 and independently by Anderson et al. in 1970 appears to be the most widely accepted explanation for the presence of CDH.14–16,58
Mechanoreceptors located at the pulp- dentin interface are theorized to stimulate the conduction of A-δ myelinated nerves to produce the painful response of CDH from stimuli. Free nerve endings have been found to extend into dentin tubules from the pulp- dentin interface by approximately 100 microns may also contribute to this neurogenic phenomenon. Their connection is to non-myelinated, smaller diameter, c-fibers of slow conductive speed.59–61 The inward and especially outward flow of dentin tubular fluid, according to this theory, results in CDH pain. The production of neuropeptides Calcitonin Gene-Related Peptide (CGRP), neurokinens A and B (NKA, NKB), and substance P within the pulp in close proximity to vascular elements, “neurogenic inflammation,” may also contribute to reducing thresholds of neural excitation.5 This theory applies when a threshold of open dentin tubules exists on the oral/salivary extent of root dentin by at least partial denudation of pellicle or smear glycoprotein/debris layers in the absence of cementum and other attached periodontal tissues. The presence of CDH requires additional study to determine if theory advanced by Brännström with subsequent investigations applies to all conditions of CDH.
Rapp, et al proposed that odontoblasts could serve as direct receptors to transmit the pain response of CDH from oral stimuli, but electron microscopy failed to find synaptic junctions between pulp nerves and these cellular elements.8,62,63 The “odontoblast transducer theory” for the pain reaction of CDH has been hypothesized, but not possible in that their presence did not extend the full length of dentin tubules to root surfaces.17 A recent publication by Chung, et al reported that molecular analysis of neural transmission finds stimulated release of neurogenic mediators in pulp tissues and odontoblasts that produce activation of mechanoreceptors as well as thermoreceptors.64 Tokuda, et al reported in their investigation that human and rat odontoblasts sense mechanical stress of dentin fluid flow in tubules to activate K+ and Na+ channels, causing the release of molecular transducers for regulation of neurogenic inflammation.65
Concepts developed by Brännström suggesting that dentin fluid flow results in neural stimulation at the pulp-dentin interface have not been disproved. Modern concepts to reduce CDH rely upon closure of root dentin tubules, de-polarization of afferents at the pulp-dentin border, hypnosis, or otherwise reducing hydraulic conductance.52,66–68 The presence of mechanoreceptors at the pulp-dentin interface as well as the more slowly conductive free nerve end extensions into dentin tubules has been extensively studied in the literature.69–73 To this day, acceptance remains for dentin tubular liquid flow resulting in stimulation to pulp receptors, producing CDH. This mechanism is supported by the treatment success of occluding tubules that were investigated using the Scanning Electron Microscope (SEM).8,27,29,74
Until recently, analysis of odontoblasts contributing to CDH pain was without foundation in that neural extensions do not extend from pulp to the cementoenamel junction (CEJ) regions.27 Neural transmission of molecular mediators produced in/by the pulp tissues can result in an odontoblast stimulation/reaction following their release from stimuli of CDH.64 This alternative theory concerning an enigma of CDH cannot be dismissed as a co-etiologic theory for this non-structural painful dental event.62
Berkowitz et al. investigated pulp nociception prior to and following placement of resin- based composites.75 Their results found a 10% increase of Appreciable Hypersensitivity (AH) at a 4-week post-restorative placement time among patients without pre-op AH evidence. Of interesting note, was that pre-op detection of visual caries was not found to produce AH. Resin-restored teeth do not have open dentin tubules to the oral environment. Bacterial acids or proteases as well as other endogenous/exogenous acidic conditions could therefore not initiate fluid flow from open dentin tubules. The presence of dentin fluid evaporation likewise could not act as a stimulus for this nociceptive condition for the same reason. The study results indicate to the clinician that DH does not necessarily result from caries. Also, the presence of DH may be related to resin placement, air entrapment, or poor bonding.
A subsequent publication by Chung, et al in 2013 discussed neurotransmitter release by odontoblasts that result in nociception or lower its induction threshold in the trigeminal ganglion and/or centrally to the medullary dorsal horn.64 These neurotransmitters are a part of the neurogenic inflammatory process which produces CDH and could accentuate conditions of DH.
A combination of the study by Berkowitz, et al and the publication by Chung, et al suggests that DH does not require open dentin tubules to initiate pulp pain.64,75 In addition, neurogenic inflammation can lower threshold induction for CDH nociception by at least partial activation at mechanoreceptor and thermoreceptor sites. These two publications suggest that odontoblasts are involved in pulp pain or the promulgation thereof. Neurogenic inflammation reporting is indicated to enhance our understanding of CDH induction.
Further study seems indicated to investigate each of the mechanisms involved in the enigma of CDH. Study of fluid flow within dentin tubules has been accepted to explain CDH induction by the hydrodynamic theory when open dentin tubules exist on root surfaces.76,77 Molecular mediator study has added substance to the odontoblast transduction theory for pain reaction.64 Additional study of the frictional event from occlusal intercuspation and transmission of temperature change to receptors is indicated.
INCIDENCE
The presence of CDH has been an enigma of pain perception/reaction among human populations for many years.17 Although we cannot determine its origin, literature review finds CDH reported for decades as a dental “sensitiveness” or hypersensitivity.1 Methodology for CDH detection has been varied, ranging from subjective patient reporting to in vivo placebo/study group investigation with “an air blast,” cold, or tactile stimuli.52,78,79 Pashley recommended in 1990 that the use of a short time duration air stimulus would reduce the outward flow of dentin fluids from evaporation within open tubules for improving CDH clinical investigation.5
This article discloses a technique using attenuated air volumes at a short specific distance for identified duration of time to offer continuity during detecting and quantifying this chronic dental pain.80 An air indexing method was developed by Coleman in 1979. When Pashley’s 1990 chapter was constructed, he was not aware of this air indexing technique until Coleman’s manuscript was near to submission toward its publication in 2000.5,80 The air indexing method was defined as: The introduction of an attenuated volume/pressure of air directed to the CEJ at a 45° angle to the long axis of a test tooth for ½ to one second with room temperature air at a distance of aproximately ½ cm. Attenuated air pressures from a standard air/water syringe were measured at 2-3, 4-6, 11-17, 25-30, or 35-40 psi. A “threshold patient response” was that least volume of air responsible for minimal nociception. Patient complaint or operator detection of one or more sensitive teeth led to investigation with the air indexing method. A diagnosis was not rendered until at least 7-10 days after its verified detection to rule out occasional incidents of etiologic conditions such as an episode of vomitus or excessive stress.
Analysis of several studies investigating the incidence of CDH finds it appears most frequently among 20 to 40-year-old populations with a mean of approximately 25-30 years.21,29,81,82 The potential for CDH occurs after alveolar dental stability during late teen years and among study populations with good oral hygiene habits. It is rarely detected among younger groups and less frequently in older individuals. This nociceptive response to stimuli has been deemed to arise as a threshold event when enough open dentin tubules exist with alveolar stability to reach CDH pain reactivity.21 Secondary or reactive dentin formation has been explained as the reason why older populations are less frequently found with CDH.76
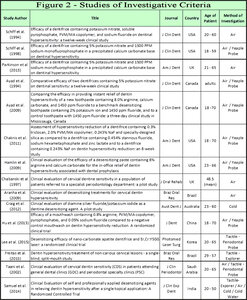
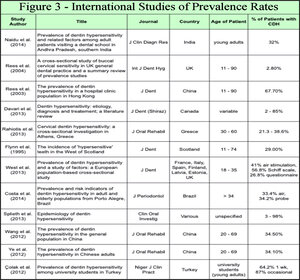
Figure 2 illustrates a selection of studies in the literature. Investigations range from reporting by Visual Analogue Scales (VAS), Verbal Rating Scales (VRS), or Schiff Cold Air Sensitivity Scale (SCASS) for detection of CDH by various air blast techniques or tactile stimulation/detection techniques with Yeaple probe, periodontal, or explorer probes.53,66,67,83–94 Studies in Figure 2 were intended to display these investigative methods, not to analyze their validity as research tools for detecting CDH. The evaporation of dentin fluids results in outflow, which stimulates nociception if a receptor threshold of pain occurs from open tubules. The mechanism advanced by the hydrodynamic theory requires open tubules for stimuli activation, so evaporation needs to be minimized during CDH investigations. Matthews, et al introduced in their 1993 study that desiccation of dentin fluids from a prolonged air blast to cervical regions may exaggerate CDH recordings during its detection.79 Reduced distance, increased air volume, and/or greater time duration of exposure to air enhances outward dentin tubule fluid flow from evaporation. Of note is that CDH is primarily detected among younger populations whereas the NCCL initiates and progresses as a process over time. Figure 2 and previous material suggest that a clinical CDH diagnosis occurs for only a few teeth at a time with air indexing and was verified later to exclude erroneous incidental etiologies.
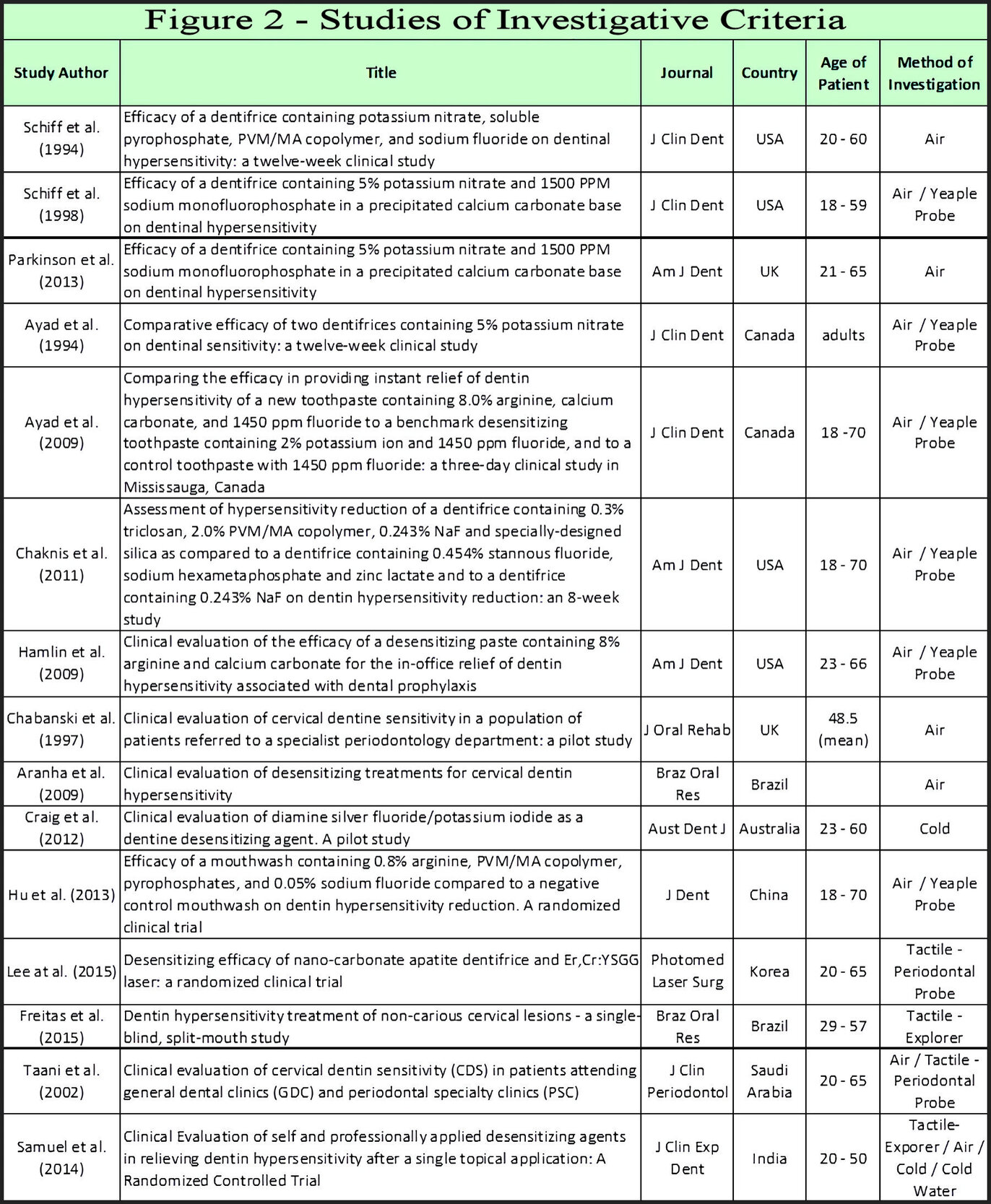
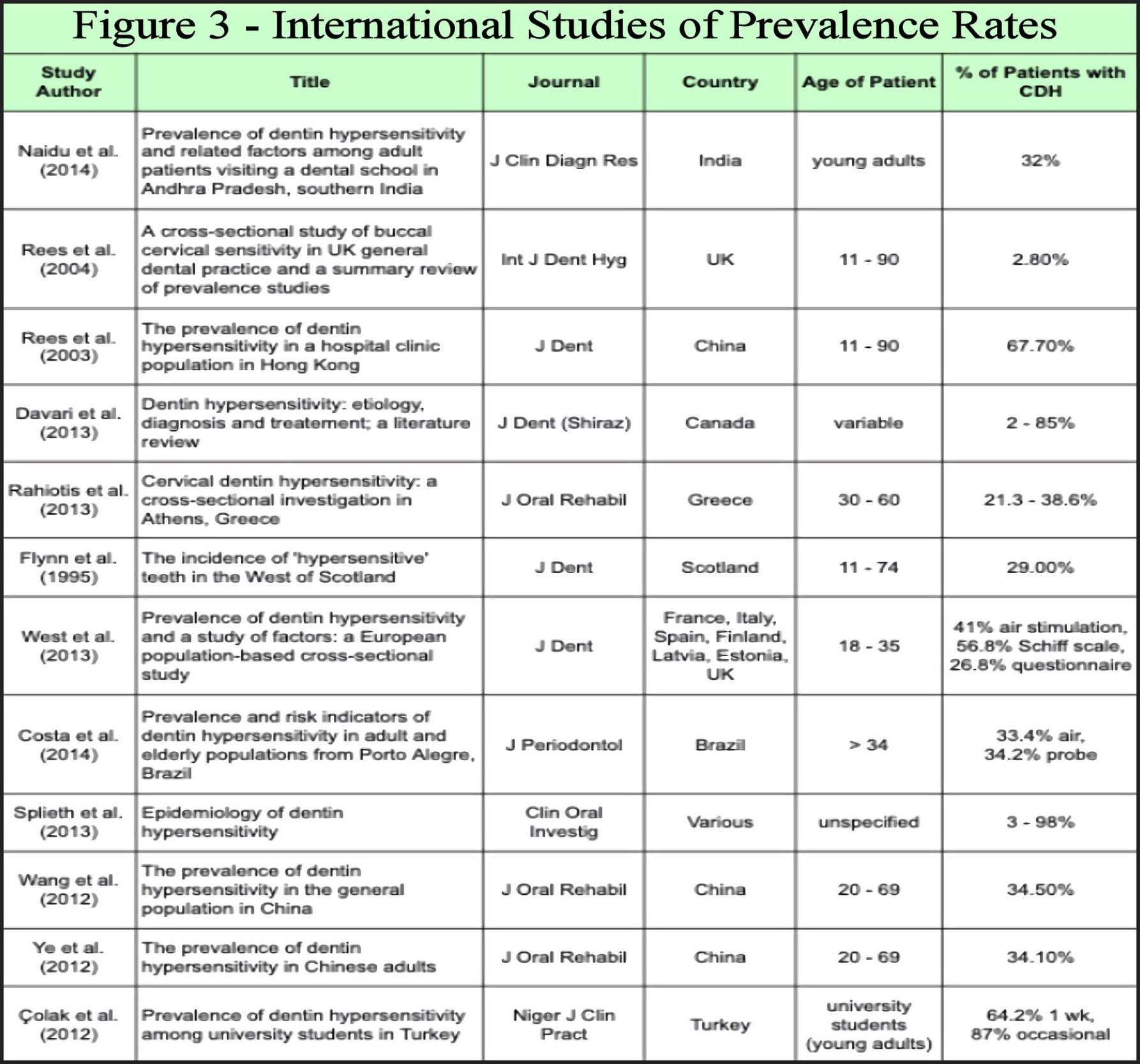
Studies found that 2-98 % of groups have been reported with CDH.30,95–105 Figure 3 illustrates reports of CDH from various global locations investigating its frequency. Acidic diets, common to modern populations, societal variations of alcohol intake, coarseness of foods, and environmental exposure to abrasives all affect biocorrosion or stress events upon the integrity of smear layer/cementum influence and frequency rates of CDH.104–107 The wide range in rates of CDH among modern populations has been investigated irrespective of these variables as well as by respective study design.68,75,95–105 Investigations have reported CDH indices using subjective patient complaints or various analytical tools to determine frequencies. Distance from air source to root, volume of air, and evaporative consequences to dentin tubule desiccation impacts its reported prevalence.5 The use of an air blast stimulus during CDH study has become an accepted non-subjective investigative means.78 It is suggested that the widespread use of over-the-counter (OTC) dentifrices and mouthwashes as well as professional treatments alter incidence reporting from available studies.108 With respect to alveolar stability, CDH studies of older age groups or adolescents yield frequency rates less than among 20 to 40-year-old populations.109 The wide range of CDH rates are therefore anticipated from studies in Figure 3.
GINGIVAL RECESSION and ALVEOLAR BONE LOSS
The finding of isolated gingival recession (GR) is often associated to the presence of CDH. See Figures 4-7 for examples of isolated recession with associated CDH. Cementum crack formation from repeated occlusal loading has been found by Noma, et al to degrade its potential for attachment to root.110 Acellular coronal cementum loss then produces isolated GR from chronic occlusal stresses. Neither “frictional events” from toothbrush/dentifrice, nor the flow of fluids across root structure seem clinically possible to initiate these events of attachment loss. Likewise, events of exogenous or endogenous biocorrosion have been shown to occur in more of a regional nature of teeth.111–115
Stress concentration to cervical regions produces an effect of fatigue degradation to dental anatomy, accentuated by biocorrosion.107,116–119 The engineering term “composite beam analysis,” discussed by Misch for implant-bone stress, applies to the tooth-bone-cementum interface in that by engineering findings, materials of different elastic moduli suffer structural loss at their junction from stress over time.120 The concept of composite beam analysis in regions of chronic stress concentration has application to varying elastic moduli, whether in regions of enamel-dentin, implant-bone, or bone-root dentin.
The principle of “biologic width,” introduced by Gargiulo, et al in their 1961 publication, disclosed that a physiologic dimension slightly less than 3 mm exists between junctional epithelium attachment height and bone.121 This dimension, occupied by collagen, blood vessels, and other supporting soft tissues, has been verified.122–124 Attachment loss, by whatever means, produces an adaptive response of axial bone loss. Interruption of cementum integrity from gingival pocket inflammation and aggressive preventive treatment methods is deemed to also result in cementum interruption. The loss of cementum occurring during cervical stress concentration from occlusal loading found by Noma then results in isolated GR.110 The adaptive consequence of biologic width maintenance may therefore be interpreted as either physiologic or pathologic over time by the clinician.
A combination of composite beam analysis and biologic width studies include clinical evidence of isolated gingival recession and accordingly, bone support loss from chronic microtrauma. Our models for alveolar/periodontal bone loss from inflammation need to include stress concentration results toward a greater appreciation/application of oral physiology and/or deemed pathology.125
DISCUSSION
We may improve our understanding of DH and CDH by recognition of differences between these two types of dental pain. Establishing a clear cause and effect relationship is paramount for clinical decisions of treatment. The presence of pain often drives the health professional toward treatment, but successful resolution requires an accurate diagnosis.
Inflammatory pain is a sympathetic vascular response of smooth muscle tone resulting in increased blood flow from irritant stimulus introduction.20 Once the instigating stimulus is removed, blood flow reduces over time and vascular pressure sensors return to a physiologic, or normal state. This type of pulp pain is generally dull and poorly localized with resolution without treatment in a week to 10 days. An example of inflammatory pain resulting from early or slowly progressive bacterial caries implies sympathetic un-myelinated C-fiber activation.63,69 Neurogenic inflammation from mediator release by odontoblasts may intensify this pain reaction and vice versa from stimulation of either C- fiber or A-δ fibers.
Dentin hypersensitivity is a pain reaction of threshold activation resulting from pulpitis (increased blood flow), thermoreceptor stimulation, odontoblast release of neurogenic mediators, or combinations of events. C-fiber activation has been implicated during this pain sensation.5 In addition, this type of pain response to stimuli may be a reflection in the number of neural receptors stimulated to produce a pain reaction from the trigeminal ganglion or centrally to the medullary dorsal horn.60,64
Cervical dentin hypersensitivity appears to develop from A-δ activation from stimuli that also result in DH. However, it has been characterized as a rapidly induced intense pain in cervical regions which is successfully treated by dentin tubule occlusion or the use of depolarizing agents.5,15,16,27,29,49,54–57 However, symptomatic treatment does not solve or address the etiological factors involved in CDH or DH.
This literature review finds that we have concentrated our efforts on treating CDH rather than investigating its etiology. Cervical stress and/or biocorrosive conditions seem to be primary causative factors for CDH.
CONCLUSION
The acronyms DH or CDH can now be used when constructing publications from research or clinicians. Both stress and biocorrosive contributions need greater evaluation for CDH pain. Additional studies are suggested to clarify etiologic factors for either condition.
External Funding
None
Potential Conflicts of Interest
None

_and_cdh_for_this_39-y.jpg)




_and_cdh_for_this_39-y.jpg)


