INTRODUCTION
Complete Implant reconstruction has become both common place and reasonably successful due to Osteointegration predictability, bone grafting techniques, and implant part manufacturing precision. Restorative success has been aided with the use of strong materials like Zirconia, and where needed because of extensive tissue loss, forgiving materials that comprise hybrid restorations. Despite the success of replacing missing teeth with implants, very little literature has assessed how patients function with fully implant-supported restorations, like when All-on-6 prostheses oppose each other.
Within the dental literature most chewing evaluations have been performed with particle size analyses, and significantly less with jaw tracking technologies that can measure chewing mandibular motion patterns. Particle size changes from chewing are somewhat interesting, but do not provide clinically relevant assessments of chewing muscular performance, chewing motion mechanics, or the effort expended muscularly to perform effective chewing. Further, particle size research is messy and solely reports on the end result of chewing but does not evaluate the chewing motion process in any measurable way.1,2
Other methods to assess chewing performance include EMG activity,3 the number of cycles required for mixing colored gum together,4 the cycle time, the Average Chewing Pattern shape, and Jerkiness.5–7 Importantly, using the biometrically derived normal Average Chewing Pattern (ACP) and the normal Average Chewing Cycle (ACC) both establish clinical baselines for evaluating masticatory function. The ACP reveals the shape, timing, variability, and smoothness of an individual’s chewing movements, while the ACC reveals the muscular hierarchy, any muscular variability, the presence of muscular Silent Periods (SP), the masseter and temporalis muscles’ timing during bolus crush, and the effort expended by individual muscles to cyclically masticate.5–7
Two recent studies involving prosthodontic tooth replacements both reported mastication measured with incisor point jaw tracking (EGN) was statistically improved when missing teeth were replaced.8,9 One study evaluated a treatment partial denture’s effect on the mastication of 38 subjects who were missing molars and premolars. The Opening Time, Occlusal Time, the Turning Point, the Terminal Chewing Position, the Velocity of Chewing, the Opening and Closing Angles, and the Jerkiness in Closing were all found to be significantly changed with a treatment partial (p < 0.05). And several muscles showed significant reductions in both timing and variability (p < 0.05).8 In the 2nd study, 30 fixed prosthodontic patients chewed gum and a hard bolus to determine changes in masticatory functional parameters towards or away from normal control group chewing values (a = 0.05). The authors reported that the installed prostheses mostly changed the mastication parameters closer to normative reference values.9
However, mastication studies of complete implant-supported restorations are nonexistent, despite the extensive emphasis restorative dentistry has placed on implant prosthetics over the last 40 years. No knowledge has been put forth as to how patients chew after transitioning from a broken-down dentition requiring complete extraction into a complete fixed-in-place implant reconstruction. Therefore, the Specific Aims of this study were to use 3-dimensional incisor point jaw tracking (Electrognathography EGN), mastication motion software, and electromyography (EMG) to assess chewing mechanics, muscle hierarchy, and the muscular effort expended when broken down dentition patients chewed both gum and a hard bolus, compared to when the same patients chewed with complete implant-supported, fixed-in-place restorations.
METHODS & MATERIALS
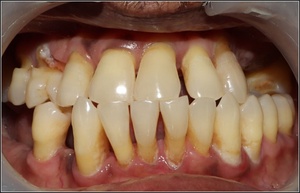
In an ongoing clinical practice of Prosthodontics, patients who presented with a failing dentition, advanced periodontal disease, and hypermobility of many teeth requiring full mouth extraction who elected to undergo fixed implant complete reconstruction were recruited for the study (See Figures 1 - 4a & 4b)
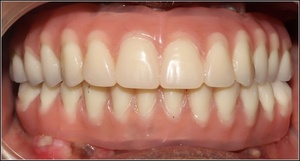
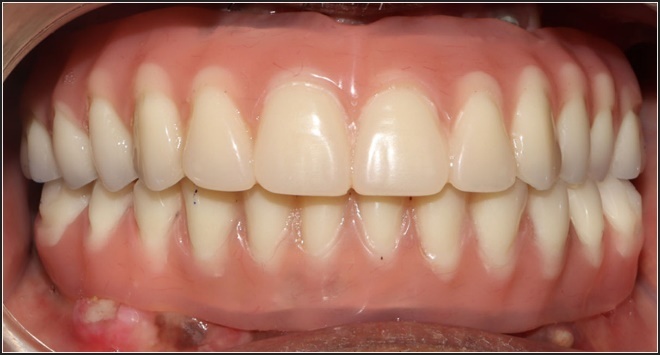
After 3-4 months, the transitional prostheses were changed into fixed-in-place hybrid prostheses (Figures 5-7).
All included subjects were measured with 3 biometric chewing technologies (EGN and EMG (Bioresearch Assoc., Milwaukee, WI, USA); T-Scan 10 Novus (Tekscan, Inc., Norwood, MA, USA)) pre and post restoration. Disclusion Time Reduction (DTR)10 computer-guided occlusal adjustments were performed on the final hybrid prostheses at their insertion.
Inclusion Criteria
-
Subjects that were either already edentulous or scheduled for total extractions, with all teeth to be replaced with All-on-6 maxillary and mandibular opposing complete implant rehabilitations
-
Subjects who agreed to accept full mouth rehabilitation with immediately loaded implant restorations
Exclusion Criteria
-
Subjects with medical conditions that prohibited needed surgical procedures and full mouth rehabilitation
-
Subjects that declined to allow their chewing data, their muscle data, and their occlusal function data to be used in a research project.
-
Patients with intact dentitions that did not require complete extractions
Ultimately, forty subjects (28 Males; 12 females) were selected for treatment from a continuous pool of prospective patients. Their pre-treatment mastication and Disclusion Time measurements were then compared to their post-treatment and post T-Scan-guided adjustment measurements using the Wilcoxon Signed-Rank test.
All participating subjects were informed that their data sets for chewing, muscle, and occlusal function would be used in a research project. Further, all extractions, bone grafts, implant placements and restorative procedures followed the recommendations of the 64th General Assembly of the World Medical Association Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects (2013). All employed restorative procedures were standard, non-experimental prosthodontic techniques.
Electrognathography (EGN) Recording Procedure
Each subject was recorded with an Electrognathographic jaw tracker while chewing gum on their left and right sides, which was repeated with a hard bolus (Peanut Chikki). Four chewing sequences with both mediums were recorded prior to any treatment being rendered. After the completion of the All-on-6 implant and restorative treatment, the same four chewing sequences were re-recorded with both chewing mediums. Mean values and standard deviations of all mastication parameters were calculated for each sequence, and compared between pre-treatment to post treatment (Alpha = p < 0.05).
During chewing, the movement of the incisor-point was captured by a magnet adhered to the lower incisors in the labial vestibule, placed low enough to avoid making any maxillary incisor contact. A magnetic sensing array (JT-3D, BioResearch Associates, Inc. Milwaukee, WI, USA) recorded the movements of the magnet vertically, anteroposteriorly, and laterally (Figure 8).
The 3-dimensional chewing data was first displayed as individual channels, but after the sequence was accepted, the displayed data was segmented into individual cycles (Figure 9).
The BioPAK software (BioResearch Assoc., Milwaukee, WI, USA) calculated the Average Chewing Pattern (ACP) of the first 15 clean chewing cycles (Figures 10 and 11).5 Each subject’s ACP was automatically compared graphically with the mean normal frontal, sagittal, horizontal and velocity motion patterns (the black ovals visible in each dimension), which are scaled to the individual subject’s vertical dimension.
Muscle Activities Recorded During the EGN Recordings
Simultaneously with the chewing recordings, the bilateral masseter and anterior temporalis muscles were recorded with the BioEMG III Electromyograph (BioResearch Associates, Inc. Milwaukee, WI, USA). Means and standard deviations were calculated for each muscle parameter and compared from pre-treatment to post treatment using the Student’s t test.
The calculated muscle function chewing parameters were:
-
Mean Area – The rms amplitude of each muscle integrated and averaged during each cycle
-
Coefficient of Variation – The standard deviation divided by the mean, which indicates muscle variability
-
The Peak Amplitude – which routinely occurs during the closure to crush the bolus
-
Time To Peak Amplitude – The time required to reach the highest value in microvolts during a cycle
-
Time to 50 % of Peak Amplitude - The time required to reach ½ the highest value in microvolts during a cycle
The recorded EMG data was displayed in the EMG RMS Sweep window as rectified averaged data, with each muscle’s individual channel superimposed (Figure 12). With healthy mastication, all 4 muscles should be functioning synchronously, but in Figure 12, cycle 11 both temporalis muscles deviated during the early part of closure (red lines). This cycle was rejected as an outlier (more than 2 standard deviations away from the mean of the sequence).
The Average Chewing Cycle (ACC) revealed the hierarchy of muscle activity (Figure 13). In normal functioning subjects the working masseter produces the highest peak activity, followed by the working anterior temporalis, the non-working anterior temporalis and lastly the non-working masseter. The working masseter provides the most activity, often producing 40% of the chewing activity, while the anterior temporalis produces 30%, the non-working anterior temporalis 20%, and the non-working masseter only about 10%. By regularly switching sides a normal subject can chew indefinitely with minimal muscle fatigue. Subjects with a malocclusion often alter their muscle pattern to adapt to the current conditions. And although successful adaptation can occur without symptoms, muscular symptoms are often associated with altered muscle hierarchy.
The calculated parameters describe the muscle activities that aid in understanding masticatory dysfunction (Figure 14). The Mean Area indicates how much each muscle is contributing to the chewing process. The SD (standard deviation of the mean) reveals any excessive variability. The CV (coefficient of variation) relates the SD to the Mean and is a better indicator of excess variability (with a normal target value of CV > 0.30). The Peak Amplitude (the maximum activity associated with the bolus crush) and its’ SD (standard deviation of the peak Amplitude), indicate chewing variability. An increased Mean Area and a larger Peak Amplitude together with a reduced coefficient of variation indicates improved masticatory muscle function.9
The Wilcoxon Signed-Rank test was applied to the 21 masticatory movement parameters of not normally distributed data (determined by the Cramer-von Mises test). And, because masticatory muscle function is synchronous among all four chewing muscles, the muscles were tested together with the Student’s Paired t-test, after verifying the normality of the muscle data (Cramer-von Mises test). Lastly, during the restorative insertion the unadjusted but installed Disclusion Time measurements were compared to the computer-guided and adjusted Disclusion Times, using the Wilcoxon Signed-Rank test.
Post Treatment Satisfaction Survey
After restorative completion, a 10-question 4-level survey was conducted to assess all subjects’ level of satisfaction. The questions related to timeliness and the meeting of expectations were scored with 4 levels of satisfaction (0 to 3). Median scores and the mean percent of the maximum score (= 3) were calculated from the entire group’s responses.
RESULTS
Kinematics
9 masticatory movement parameters changed significantly after the implant restorative treatment was finalized (p < 0.05). See Tables 1 and 2.
-
Turning Point - Antero-posterior
-
Turning Point - Antero-posterior Standard Deviation
-
Turning Point - Lateral Standard Deviation
-
Terminal Chewing Position – Vertical Standard Deviation
-
Terminal Chewing Position – Antero-posterior
-
Maximum Lateral Width
-
Maximum Lateral Width - Standard Deviation
-
Maximum Opening Velocity
-
Maximum Closing Velocity
In addition, 4 other parameters suggested there was a trend towards significance (p < 0.01).
-
Opening Time
-
Opening Jerkiness
-
Opening Jerkiness - Standard Deviation
-
Turning Pont - Lateral
For brevity, only the data from right-sided gum chewing and left-sided hard bolus chewing are presented. The Wilcoxon Signed-rank test of soft gum chewing produced significant changes in 7 mastication parameters (p < 0.05), with trends towards significance in 3 more parameters (p < 0.10). One case was removed from the group due to missing data. See Table 1.
The Wilcoxon Signed-Rank test of hard bolus chewing (Peanut Chikki) indicated significant improvements in 7 of 10 post treatment parameters (p < 0.05). 3 other parameters trended towards improvement (p < 0.10). Two cases were removed due to missing data.
Muscle Activity
Concurrent with the improved chewing movements, significant changes occurred in the muscle activities post treatment. Within the Right-side Gum chewing data, 3 of the 5 muscle parameters were significantly changed, indicating the chewing motion improvements resulted from changed muscle activities (p < 0.05). See Table 3.
The left-sided Hard Bolus data revealed significant changes in 4 of the 5 muscular parameters (p < 0.05) with a trend towards significance in the 5th parameter (p < 0.10). As the hard bolus required more mastication muscular effort, greater changes in the muscle function were seen post treatment. See Table 4.
There was a statistically significant reduction in the muscular effort expended by the subjects as they chewed. The contraction durations per muscle during excursions was shorter after the restorations were placed and occlusally adjusted with the T-Scan/EMG (See Table 5).
Prosthetic Occlusal Contact Finishing
The final step in the delivery of the implant-supported restorations was the measurement of, and then the computer-guided adjustments made to the excursive Disclusion Times. The T-Scan 10/BioEMG III synchronization directed the establishment of short Disclusion Time (< 0.5 seconds per excursion) on the occlusal surfaces of the implant restorations (Figures 15 and 16).
The pre-T-Scan adjusted Disclusion Time measurements were compared to the post-treatment T-Scan adjusted Disclusion Time measurements using the Wilcoxon Signed-Rank test. The changes in the Disclusion Times were significant (p < 0.00001) (see Table 6).
Patient Satisfaction Survey
The post-treatment ordinal number survey consisted of 10 questions that were subjectively responded to by all 40 subjects. Overall, the implant reconstruction treatment had been highly satisfactory (see Table 7). The median score in 9 of 10 questions were scored by subjects at the maximum of 3. The mean percentage of the maximum score for all questions was 91.75%, indicative of very high levels of satisfaction with the treatment process and the completed treatments.
DISCUSSION
The findings of this mastication implant research are unique in that to date, there are no pre-existing studies that have reported on measurable chewing efficiency improvements with implant restorations. However, the Results do corroborate other EGN and EMG chewing studies where the occlusal contacts were optimized with T-Scan/EMG data to improve chewing strength, chewing speed, cycle timing while reducing variability.6–9,11 Another reason this study’s findings are unique is that two different boluses were compared for observed chewing improvements. The hard Peanut Chikki challenged the subjects more than did the soft gum, yet the subjects as a whole illustrated chewing improvements regardless of bolus consistency.
Although the chewing improvements in most parameters were statistically strong, it could be misperceived that these chewing changes resulted “just because fixed in place teeth replaced broken down dentitions”. It’s important to realize that fabricating the macro-occlusion (the All-on-6 prostheses) that was done from the digital restorative design with CAD/CAM machining, did not guarantee the fabricated macro-occlusal contacts operated optimally. Studies continue to show that despite the Digital Workflow, occlusal complications and breakage problems persist in implant dentistry, of which one of the reported complications is patient occlusal discomforts.12–17Although in this study no complications were tracked, the parametric chewing improvements resulted not only because implants were placed strategically and restored digitally, but because T-Scan/EMG force and timing data sets guided the occlusal adjustments made to both the transitional and final restorations.
Computer-guided occlusal optimization of contact forces, contact time simultaneity, and excursive Disclusion Time durations have been shown in many TMD and occlusal function treatment studies to create physiologic muscular function at the tooth contact level.18–24 In this mastication implant study, the application of those same computer-guided occlusal function controls made at prosthesis delivery, directly aided the subjects’ ability to chew with their new All-on-6 implant restorations.
Importantly, none of this study’s measured mastication improvements in strength, duration, motion pathways, and cycle timing could have been detected without the series of biometric technologies that assessed patient function. The idea that biometric instrumentation has “little merit” in the study of human occlusion and human chewing capability, sometimes being referred to as "gadgets,25 or that the weak, dysfunctional chewing seen with TMD patients is a problem stemming from “a patient’s compromised emotions26” are both unfounded opinions.27 Clearly, this study showed that multiple biometric measurements all utilized in a coordinated, organized restorative approach, precisely detected patient chewing motions, the muscles’ physiology during chewing, and optimized the occlusal contact timing that influenced functional chewing, from which the treated patients were enabled to chew with measurable physiologic improvements.
Of note is the muscular effort expended in chewing from prior to restoration compared to after restoration (Table 5), where between C - D in the muscular EMG data, there were statistically significant reductions in the effort expended by all four muscles (Figures 15, 16). The reported values in Table 5 are the amplitudes multiplied by the time duration each muscle-maintained contraction during excursions (microvolts x seconds). The values indicated the relative amount of effort expended during the transition from leaving MIP (C) to when posterior disclusion measurably occurred (D) were markedly reduced, while the contraction intensity typically was similar. This reduction in muscular effort was directly tied to creating short Disclusion Time (Figures 15 and 16; see Table 6) when the prostheses were installed. This illustrated the functional value of installing complex implant reconstructions with T-Scan 10 computer-guided force and timing data.11 Muscular physiologic control and hierarchy synchronicity during chewing has been previously shown to vastly improve when short Disclusion Time is incorporated into the occlusal surface functional contours.6–9
Lastly, Table 7 indicated that being converted from a failing dentition into an esthetically restored patient with a full complement of well-functioning, fixed-in-place teeth was quite an improvement for the 40 subjects. The post treatment satisfaction Means were unanimous for all subjects, while with the ordinal number Medians suggested all questions but number 3 (Did your treatment plan coincide with your wishes?), might have been unanimous for all subjects.
CONCLUSION
Implant-supported prostheses can measurably improve masticatory function when precisely constructed, and installed with computer-guided force and timing-controlled insertion occlusal adjustments. Complete implant-supported reconstructions that are digitally-planned for navigated implant placement surgery, and digitally-designed for optimal prosthetic tooth replacements, are functionally aided by digitally-guided occlusal adjustments made at case installation.
DISCLOSURE
Roshan P. Thumati practices Orthodontics and Dentofacial Orthopedics, COPE Dental Health Care Center, Banashankari, Bengaluru, India.
Prafulla Thumati is professor of prosthodontics at Rajarajeswari Dental College and Hospital, Bengaluru, India.
Robert B. Kerstein is a former Assistant Clinical Professor at Tufts University School of Dental Medicine.
John Radke is the Chairman of the Board of Directors of BioResearch Associates, Inc., Milwaukee, WI, USA.
Funding
This research project received no funding from any corporation involved in the manufacture of the utilized technologies.







._the_jt-3d_se.jpeg)

_and_blue_(closing)_colored_line.jpeg)


_reveals_the_hierarchy_of_masseter_and_anterior_temporalis.jpeg)











._the_jt-3d_se.jpeg)

_and_blue_(closing)_colored_line.jpeg)


_reveals_the_hierarchy_of_masseter_and_anterior_temporalis.jpeg)


