INTRODUCTION
Trigeminal Neuralgia (TN) is a well-known orofacial pain condition that remains the most common of the cranial neuralgias.1 It is also referred to as “The Suicide Disease” and Tic-Douloureux. TN is clinically diagnosed from symptom presentation, which is followed by an MRI to confirm the diagnosis. TN affects 4-5 persons per 100,000 each year, mainly affecting patients of 50 years and older, but anyone can be diagnosed with TN.2 TN presents with a characteristic trigger zone that elicits intense shooting pain. The response, often referred to as an “attack” or “flare up,” is uncharacteristically intense compared to the stimulus. Activities such as brushing teeth, shaving, eating, or a cold wind blowing on the face have been known to set off a TN attack.1
According to Burket’s Oral Medicine, “A diagnosis of TN is usually based upon a history of shooting pain along a branch of the trigeminal nerve, precipitated by touching a trigger zone. A TN diagnosis can be made from an examination that demonstrates the shooting pain.”1 The exact cause of TN remains debatable, but approximately 10% of the cases present with a verifiable pathosis, such as vascular malformation or tumor. The most commonly accepted TN etiology is vascular compression and demyelination of the trigeminal nerve root. Unfortunately, no other description has been offered, with 90% of all cases being deemed idiopathic.1
Historically in 1910, Cushing first described a “cranial nerve vascular compression syndrome,”3 where a sixth cranial nerve palsy resulted from increased intracranial pressure in the posterior arteries that stretched the nerve. Dandy extrapolated this concept to cranial nerve 5. He published the surgical results of treating the trigeminal nerve sensory root of two TN patients,4 and subsequently operated on 215 TN patients, cataloguing each case’s causative etiology.5 Tumors accounted for 5.6%, arterial nerve compression for 30.7%, venous nerve compression for 14%, but in 40% of the patients “no gross findings were observed” (the largest single category). Gardner et al. attempted to verify Dandy’s observations, but the authors found vascular compression was present in less than 50% of his patients.6–10
In a 1993 manuscript entitled Vascular Compression is the cause of Trigeminal Neuralgia,11 Janetta wrote: “It is a central truth of science and medicine that ideas that precede the technology to prove or disprove them, lie fallow,” claiming the initial findings of the TN pioneers lacked magnification capabilities, where the nerve roots could not be completely examined.11 Jannetta advocated that neurovascular compression was the primary etiology of TN, despite reporting on 10 microvascular decompression (MVD) studies12–21 where incomplete relief, or TN symptom recurrence was observed in 4-32% cases, and negative MVD findings observed (no compression found) in 2-16% of these same subject groups. Confounding things further, multiple MRI studies analyzing both TN patients and non-TN control patients found neurovascular compression present in the non-TN control group, while some TN affected subjects presented without neurovascular compression.22–26 These same studies concluded that successfully using MRI findings to guide both surgical and non-surgical treatments for TN, ranged downward from 100 - 47%. Furthermore, two of these studies reported neurovascular compression (NVC) was present on the non-TN (unaffected) side of the face in 43%-76% of the patients, with false positives (NVC but no TN pain or diagnosis) being reported 15-56% of the time.22–26
Cadaver studies performed on people known to not have had TN, where 50 trigeminal nerve roots were examined, determined that 60% of these non-TN patients had unilateral direct vascular contact with the nerve tissue, and 20% had bilateral.27 Interestingly, some of the cadavers had contact force sufficient to cause an indentation of the nerve tissue.27 A second cadaver study reported in 7-12% NVC was observed in patients with no history of TN.28 Lastly, it has been demonstrated that TN can occur and reoccur in the absence of neurovascular compression,29 and that an estimated 99.94% of individuals with a neurovascular compression do not exhibit TN.29
Current TN treatment modalities include medications (usually the first attempt), with Micro-Vascular Decompression (MVD), and gamma knife surgery,30,31 as surgical options which have demonstrated some success at treating TN, but not the extent one would expect if neurovascular compression was the only etiology. Figure 1. Moreover, these treatments carrying significant adverse effects patients must weigh before undergoing therapy. As an alternative to surgical intervention, Botulinum Toxin-A (BTX-A) is also being used for TN treatment32 as well as with other chronic pain patients and conditions33–35 (myofascial pain dysfunction,36 painful diabetic polyneuropathy, post herpetic neuralgia (PHN), complex regional pain syndrome, and neuropathic low back pain.37 The contractility of the targeted muscles is reduced as the administration of Botox therapeutically blocks the pre-synaptic cholinergic nerve terminals. There is additional speculation that Botox might have an anti-nociceptive affect that has yet to be proved out definitively.38,39
Given that the data does not clearly support neurovascular compression as being the sole etiology of TN, medicine and dentistry must hold other potential etiologies as equally possible. Dental Occlusion, which has never been proposed in the TN literature to be a possible TN etiologic contributor, was previously found in a case report to be a significant factor in both chronic orofacial pain and TN symptoms.30 Both pain conditions were resultant from bite force and bite timing abnormalities, that were resolved with the Immediate Complete Anterior Guidance Development (ICAGD) measured occlusal adjustment procedure.40 ICAGD reduces the frictional contacts of the posterior teeth that occur during lateral excursive movements, to establish measurable immediate posterior disclusion of the molars and premolars in < 0.5 seconds per excursion.40–48 The metric that determines how well ICAGD has been performed is the Disclusion Time (DT), which is the elapsed time (in seconds) between the start of a lateral excursion and the time-moment complete working side and non-working side molar and premolar disclusion is reached. The reason short DT is critically important, is because DT below 0.5 seconds have repeatedly been shown to markedly lessen excursive function elevated muscle hyperactivity.40–48 Over the past 30 years, this process known as Disclusion Time Reduction (DTR) via the ICAGD computer-guided coronoplasty, has successfully treated occluso-muscular TMD symptoms in multiple published studies, that were performed by different researchers who all used the same ICAGD process, and obtained similar therapeutic results.40–48
Objectives
Therefore, the objectives of this cohort study were to perform the ICAGD coronoplasty on a small group of confirmed TN patients whom presented with long Disclusion Times, high excursive muscle activity levels, and/or a bite force imbalance, all of which could promote TN episodes. The results of this Pilot Study will either corroborate or contradict the prior case report’s observed TN symptom reductions that followed the measured occlusal adjustment therapy.
METHODS & MATERIALS
Twenty-nine patients previously diagnosed by a primary care physician with Trigeminal Neuralgia (TN) that was confirmed by a neurologist, were evaluated in a dental practice that offers specialized Disclusion Time Reduction TMD services. All patients had prior magnetic resonance imaging (MRI), which confirmed neurovascular compression of the trigeminal nerve. Nine of the 29 prospective candidates had been treated with an accepted TN treatment; six had undergone MVD surgery, and three were treated with Gamma Knife surgery. However, neither the surgical NVC corrections, nor the irradiation of CN V’s ganglia were effective, as those nine patients never saw their pain leave, or their pain returned “full force” shortly following the procedures. Two other potential subjects were eliminated for not meeting the inclusion criteria (below), and two more patients did not complete their course of ICAGD, leaving a total of 25 study participants. An IRB exemption was requested and obtained for a retrospective cohort study #BIRB/94Z/2019.
Inclusion Criteria
-
A TN diagnosis from a neurologist with MRI indicating existing neurovascular compression of Cranial Nerve V
-
The existence of ongoing TN symptomatic episodes
-
28 teeth with symmetrically missing teeth (if one molar was missing on the left side, then one had to be missing on the right side)
-
Near normal occlusal relations with molars and premolars in contact during the right and left excursions
-
Angles Class I and Class III occlusal relations, with guiding anterior teeth that were either in contact, or near to contact
-
Patients that had been previously treated with MVD and/or Gamma Knife surgery but did not receive TN symptom resolution
-
Patients older than 18 years of age
Exclusion Criteria
-
Severe Class II and Class III malocclusions and anterior open bite where anterior guidance contact could not be achieved
-
A previous history of TMJ trauma
-
The presence of unstable Temporomandibular Joint internal derangements verified by CBCT and/or Joint Vibration Analysis (JVA).
-
Patients that had been previously treated with MVD and/or Gamma Knife surgery that received TN symptom resolution
-
Patients who had undergone prior TMD therapy, including prior occlusal adjustment treatment.
-
Patients younger than 18 years of age
Informed consent was obtained for undergoing the ICAGD coronoplasty, and for collecting pain symptom severity and frequency data from questionnaires. The study protocol required the participants to fill out questionnaires that detailed TN symptoms, chronic muscular symptoms, pain intensity and frequency, functional restrictions, and a 15 item Patient Health Questionnaire (PHQ-15), which is a validated psychiatric instrument used to detect a Somatic Symptom Disorder (SSD).49–51 The PHQ-15 detects the presence of somatization, and has been recommended for use by the DC/TMD for patient assessment in dental practices treating TMD.52 Oral health histories were also obtained where the whole participant group reported experiencing a large number of TN symptoms as well as TMD symptoms with moderate to severe frequencies and intensities. The TN symptom every participant shared was a trigger zone in the CN-V2 or CN-V3 region that when touched could set off electric or stabbing pain as well as facial pain. The TMD symptoms seemed more random and no correlation could be made from any one symptom to the TN trigger zone symptom.
All participants underwent a pre-ICAGD right and left excursive Disclusion Time/muscle hyperactivity evaluation with the synchronized T-Scan 9/BioEMG III technologies (Tekscan Inc., S. Boston, MA USA; Bioresearch Assoc., Inc. Milwaukee, WI, USA) (Figure 2). The subjects closed firmly into their Maximum Intercuspation Position (MIP), held their teeth together for 1-3 seconds, and then completed a single direction right (or left) excursion until only their anterior teeth were in contact. They repeated the recording in the opposite direction to generate 2 separate pre- ICAGD excursive recordings. This specific recording method insured high quality Disclusion Time and EMG data was obtained from all subjects.40
Additionally, the subjects had their pre-ICAGD right side-to-left side Bite Force imbalance reported in percentages (Figure 3), and their pre-ICAGD Disclusion Times reported in seconds (Figure 4), both to be compared to their post ICAGD values.
Description of the ICAGD Occlusal Adjustment Procedure
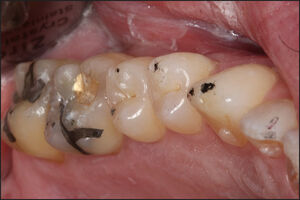
The teeth were dried on 1 side of the arch (operator’s selection), after which each subject closed into their Maximum Intercuspal Position (MIP) with 21 micron-thick articulating paper (Accufilm, Parkell, Inc. Farmingdale, NY, USA) interposed between their teeth, and to then move their mandible into a right excursion out to edges of their right canine teeth, then slide back into MIP, and then move out to edges of their left canine teeth. With this movement the teeth made excursive track lines that marked the long Disclusion Time contacts on the frictionally involved teeth for adjustment (Figure 5).
The pre-ICAGD T-Scan/BioEMG recordings guided the author to the proper areas of the occlusal surfaces that required excursive adjustments. All working and non-working posterior lateral interferences that represented the prolonged frictional contacts were removed completely, while the centric stop contacts were left intact except to lessen their broad surface area into small surface area contacts, located on supporting cusps and in central fossae. This process was repeated on the initial side of treatment until complete visual disclusion had been obtained (Figure 6). The same process of marking the teeth excursively and removing all working and non-working posterior lateral interferences was then performed on the 2nd side of the arch, until complete visual disclusion had been obtained on the 2nd side.
ICAGD was considered completed.
-
all lateral posterior excursive interferences had been visually removed,
-
disclusion of all posterior teeth in the right and left excursions afforded the patient noticeably easier lateral movements than pre-ICAGD,
-
the remaining pattern of habitual closure contacts were located solely on cusp tips, in fossae, and on marginal ridges,
-
any patient self-closure into MIP rapidly rising forces detected by the T-Scan, were refined to achieve measurable bilateral simultaneous force rises of moderate contact force (Figure 7).
-
the Disclusion Times had been measurably reduced to < 0.5 seconds in each excursion
At this same treatment appointment, new post ICAGD excursive recordings were obtained in the same manner as pre-ICAGD, to verify the Disclusion Time durations were correct. Any remaining prolonged excursive contacts were adjusted until each excursion’s Disclusion Time was less than 0.5 seconds (Figure 8).
Patients were seen at Day 1, at 1-month, and at 3-months to undergo refinements to the above procedure, to fine tune the MIP occlusal contacts, improve the Disclusion Times if needed, and to allow time for the muscles to heal from the rendered occlusal refinements. At each of these follow-up visits, the patients filled out new questionnaires regarding their symptoms, pain intensity and frequency, functional restrictions and their PHQ-15 somatization resultant from undergoing ICAGD. All subjects’ self-assessment data were subjected to the non-parametric Wilcoxon Signed-Rank Test. The Disclusion Time values and EMG levels pre and post ICAGD were subjected to the Students paired t-test (Alpha = 0.05). This concluded both the treatment and data collection phases of the study.
RESULTS
The mean age of the TN subjects was 43.8 +/- 14.87 (84% (21) female and 16% (4) male). There was some randomness to the group in that consecutive patients were treated as they presented to the practice. This resulted in an unequal distribution of the sex and age demographics, which had no observable effects on the Results.
The TN trigger zone ultimately resolved in all participants to the point that the electric shocks/stabbing pain ceased. A few patients reported the trigger zones moved from V2 to V3 (or vice versa), or moved from a position that was external to the mouth to internal. Three patients reported this finding, 2 were next to the left maxillary second premolar and 1 was to the right mandibular premolar. These were transient and resolved in a few weeks to months. Patients also reported unsolicted improvements in follow up appointments such as: “I can brush my teeth without pain”, “I can walk down the frozen food isle at the store with no scarves on.” “I went running outside even when it is cold without being covered up”, and “I can shave my face without fear of getting shocked.”
The study participants reported higher pre-ICAGD self-assessment means and medians for their TN pain level intensity, pain frequency, their functional restrictions, and significantly higher PHQ-15 scores compared to their post-ICAGD responses (Table 1).
-
There were dramatic statistically significant reductions in the TN pain severity and episode frequency means at 1-month after ICAGD (36 +/- 14.3 days; p < 0.00001), with statistically significant further pain severity and frequency improvements observed at 3-months (88 +/- 13.6 days; p < 0.00001). Table 1.
-
There were statistically significant improvements in mean functional restriction scores at 3-months post-ICAGD (p < 0.0004).
-
There were statistically significant reductions in the group’s PHQ-15 mean scores at 1-month and 3-months post ICAGD (p < 0.0012). Table 2.
-
The self-reported TN and other symptom improvements were obtained solely because there were statistically significant time-duration changes made to the pre-ICAGD Disclusion Times by the ICAGD coronoplasty (p = 0.0001). Table 3.
-
There were statistically significant reductions in the group muscle activity level (EMG) means for all 4 muscles post-ICAGD compared to pre-ICAGD, that coincided with shortening the Disclusion Times (p = 0.00001). Table 4.
The pre-ICAGD group mean PHQ-15 score =13.2 +/- 4.84, with a range of 6 - 23, suggesting that the 18 of the 25 subjects that scored higher than 10 likely exhibited a faux in retrospect SSD condition prior to receiving ICAGD treatment. The remaining 7 subjects scored a faux in retrospect “Other neurotic Disorder.” (Table 2). However, only one month after initial ICAGD treatment, the subject group’s PHQ-15 mean score statistically significantly dropped = 7.2 +/- 3.79 (p = 0.00001), which mean score (between 5 and 10) suggests a lesser neurotic symptom. At three months post ICAGD the subjects’ group PHQ-15 mean score dropped further to 4.5 +/- 2.89, which was significantly lower than the one month mean (p = 0.00118). At the three-month follow-up only one subject still scored a 12, but with numerous physical conditions complaints outside of the orofacial area. Six subjects scored between 5 and 10 and fully 18 subjects scored below 5.
DISCUSSION
The outcome of this computer-guided occlusal adjustment Trigeminal Neuralgia study contradicts all of the previous articles where it has been concluded that the physical symptoms of TN are secondary to Cranial Nerve V compression from its surrounding vasculature.31,32 This is the 2nd publication to show reducing the Disclusion Times of TN patients’ lateral excursions can rapidly and definitively lessen the frequency and intensity of TN bouts. However, this is the 1st research publication to show that Disclusion Time Reduction (DTR) with the ICAGD coronoplasty can successfully treat a group of chronic TN sufferers. The Results of this study would suggest that TN is actually subset of TM Disorders, because this study’s findings corroborate the successful TMD muscular symptom outcomes of many prior published DTR/ICAGD studies that have been in the literature since the early 1990s all the way up to 2020.40–48,53
The findings of this study also bring into question that NVC is the sole etiology for TN symptoms, while supporting other investigators who have determined in autopsy that neurovascular compression can occur in patients with no history of TN.54 Kalia suggested in 2014, “surely there are undiscovered factors in the TN story” and “perhaps the challenge is to find yet another cause of TN.”55 The fact that a number of the subjects had noticeable TN improvement or resolution from ICAGD, despite attempting multiple prior surgical treatments, adds significant credibility that the occlusion was etiologic for their TN conditions. And although ICAGD is an irreversible procedure that sacrifices about 120-180 microns of enamel (out of an existing 2000-2500 microns per tooth) to calm down the muscle hyperactivity and improve the neurophysiology, ICAGD has a long track record in studies of producing successful Occluso-muscular Dysfunction (OMD) symptom resolutions without requiring splints or orthotics, and without marked post treatment complications.40–48,53 From a contextual point of view ICAGD is a conservative treatment, especially when compared to MVD or Gamma Knife surgery (Figure 1). Therefore, it would be prudent that occlusal force imbalances and long Disclusion Times be ruled out as being causative for TN, before encouraging MVD or gamma knife surgery as a treatment option.
The reduction in muscle hyper-activity that was observed in this study must be considered as a contributory component of patients reporting symptom improvement, which corroborates the findings of prior ICAGD and DTR studies, in which when muscle hyperactivity was reduced, sustained relief followed without subjects requiring multiple retreatments.40–48,53 In that way, DTR treatment is self- limiting, and demonstrated long-term symptom control.56 And while there is great evidence showing Botulinum Toxin (BTX-A) is efficacious in treating TN,32,57–60 the BTX-A refractory period is short (3-6 months), requiring re-dosing the patient repeatedly to re-control the symptoms.
There are over 40 different diagnoses that fall under the TMD umbrella, where routinely there is a component of muscular pain. Muscle compensation is the body’s first adaptive mechanism to all structural shortcomings. Although Botulinum Toxin (BTX-A) has been used with Trigeminal Neuralgia patients32,57–60 to therapeutically block the pre-synaptic cholinergic nerve terminals and reduce muscle contractility, the results of this study suggest that the pathophysiologic mechanisms of TN must be reconsidered. In this subject group, elevated masticatory muscle activity levels were statistically reduced through computer-guided occlusal alterations, that also reduced TN pain frequency and intensity, while simultaneously improving muscle function. This was accomplished in the absence of any BTX-A administration, which supports the position that there is muscular component to idiopathic TN, which to date has been largely overlooked. TN in this subject group likely consisted of a muscular structural problem with shooting nerve pain as a secondary symptom, because a structural correction/alteration (occlusal adjustment to the excursive friction) relieved the muscle and/or nerve pain. BTX-A represents a symptomatic treatment, rather than a corrective therapy to the underlying malocclusion problem.
Of note is that in this TN/ICAGD study, the PHQ-15 scores statistically improved, corroborating a prior emotional assessment DTR study that showed the emotional states of TMD sufferers were improved after undergoing ICAGD.61 As occurred in that study of 83 emotionally affected TMD sufferers whose depression score improved rapidly after ICAGD,61 the TN subject group in this study also rapidly emotionally responded once their chronic TN pain was removed by ICAGD. For the 25 subjects in this TN study, the PHQ-15 pre-ICAGD scores indicated 18 subjects exhibited a possible somatoform disorder, and the remaining 7 subjects exhibited a "neurotic disorder." As the T-Scan-guided occlusal adjustments were performed, and the subjects’ painful symptoms were reduced, their PHQ-15 scores receded. At 1-month post ICAGD, only 4 subjects exhibited scores indicative of a somatoform disorder, 12 scored as other neurotic disorder, and the remaining 9 subjects’ PHQ-15 scores indicated they had fully recovered from their previous emotional disorder.
At three months, only one of the 25 subjects’ PHQ-15 scores was marginally suggestive of a possible somatoform disorder. However, that subject retained painful physical symptoms that (based upon the response of the other 24 subjects to the ICAGD treatment), did not appear to be related to the masticatory system. Six other subjects’ scores reduced to the level of other neurotic disorder, and the eighteen remaining subjects’ PHQ-15 scores indicated they had fully recovered emotionally. Considering that only occlusal adjustments were performed and no psychological counselling was a part of the treatment, it appears that the initial PHQ-15 scores were representing both false positive SSD or other neurotic disorder diagnoses based upon the significant secondary emotional distress present. As has been previously noted,61 the emotional recovery can take several months for a patient recovering from severe chronic physical pains.
This study’s outcome demonstrates the high risk of obtaining false positive diagnoses when testing for SSD, prior to the elimination of any existing physically painful conditions. The absolute requirement of establishing an absence of physically painful conditions before establishing SSD diagnosis is well understood by psychiatry,62–64 but seems often misunderstood by dentistry. While it cannot be concluded from this study that the T-Scan guided occlusal adjustments cured any SSD or other neurologic disorders, the well-known fact that chronic pain from any physical source predictably causes emotional distress, is not even debatable. This study’s results exemplify why an emotional evaluation cannot result in a valid psychiatric diagnosis, in the presence of physically driven chronic pain. Use of the PHQ-15 should follow the verification that all physical factors have been accounted for there, such that no physical etiology for symptoms is present. This is an important requirement that must precede any accurate diagnosis of emotionally driven symptoms.
Most of the TN literature contains substantial variation in agreement to the neurovascular compression explanation of TN. Obtaining an accurate diagnosis of neurovascular compression TN, is complicated because the anatomic appearances of neurovascular compression must be confirmed by MRI. However, multiple publications indicate that MRI may not be able to accurately detect if a structural nerve problem exists,22–26 which limits MRI from being an effective TN diagnostic image. Clinicians could be aided in making accurate TN diagnoses by requiring that TN patients fill out a TMD signs and symptoms questionnaire (See Appendix). If there are additional chronic muscular symptoms present along with the intense TN electric shock, the TN patient may be suffering from (OMD), which is one form of TMD. This was clearly the scenario for the 25 TN patients in this cohort study.
LIMITATIONS
Despite the very small P values reporting a high-level statistical significance in the TN symptom improvements, the Disclusion Time changes, and in the emotional PHQ-15 improvements observed, there were a number of limitations to the study design. First, there was a small sample size of 25 subjects, so large-scale TN symptom improvement extrapolations should not be made. A second limitation was that subjects were their own controls and not statistically compared to a separate TN subject control group. This should be done in a future study where ½ the TN patients receive a mock ICAGD procedure and the other ½ receive true ICAGD. As this specific study attempted to determine a measured treatment effect (changes TN symptoms after ICAGD), using the subjects as their own controls was necessary. To compensate for this limitation, many self-report questionnaires were used to determine pre to post ICAGD TN symptom improvements.
CONCLUSIONS
Twenty-five Trigeminal Neuralgia subjects experienced reductions in TN episodic frequency and pain severity, while functionally improving with much lower the levels of muscle activity following having their Disclusion Times shortened with the ICAGD coronoplasty. The subjects’ PHQ-15 scores also significantly reduced as their chronic pain levels were reduced. This points to the occlusion as being the primary causative agent for the symptoms in this group of TN patients, despite that the occlusion has been overlooked in the TN literature as being a potential TN etiologic component. Disclusion Time Reduction using ICAGD should be considered as an alternative treatment option for those patients diagnosed with painful Trigeminal Neuralgia. A second conclusion from this research is that emotional responses can be secondary to actual physically painful conditions and that the emotional factors can return to normal after the physical conditions have been corrected and the physical pain is relieved.
Statement of possible conflicts of interest
Dr. Ben Sutter claims no conflict of interest. Dr. Susan Teragawa claims no conflict of interest. John Radke is the Chairman of the Board of Directors of BioResearch Associates, Inc., the manufacturer of the BioEMG III. He receives no commission or other monetary incentive from the sales of the T-Scan or the BioEMG III.
Statement of funding
No funding from any source was provided for this study.





_after.jpg)









_after.jpg)



